Abstract
We present a new micromixer based on highly magnetic, flexible, high aspect-ratio, artificial cilia that are fabricated as individual micromixer elements or in arrays for improved mixing performance. These new cilia enable high efficiency, fast mixing in a microchamber, and are controlled by small electromagnetic fields. The artificial cilia are fabricated using a new micromolding process for nano-composite polymers. Cilia fibers with aspect-ratios as high as 8:0.13 demonstrate the fabrication technique's capability in creating ultra-high aspect-ratio microstructures. Cilia, which are realized in polydimethylsiloxane doped with rare-earth magnetic powder, are magnetized to produce permanent magnetic structures with bidirectional deflection capabilities, making them highly suitable as mixers controlled by electromagnetic fields. Due to the high magnetization level of the polarized nano-composite polymer, we are able to use miniature electromagnets providing relatively small magnetic fields of 1.1 to 7 mT to actuate the cilia microstructures over a very wide motion range. Mixing performances of a single cilium, as well as different arrays of multiple cilia ranging from 2 to 8 per reaction chamber, are characterized and compared with passive diffusion mixing performance. The mixer cilia are actuated at different amplitudes and frequencies to optimize mixing performance. We demonstrate that more than 85% of the total volume of the reaction chamber is fully mixed after 3.5 min using a single cilium mixer at 7 mT compared with only 20% of the total volume mixed with passive diffusion. The time to achieve over 85% mixing is further reduced to 70 s using an array of eight cilia microstructures. The novel microfabrication technique and use of rare-earth permanently-magnetizable nano-composite polymers in mixer applications has not been reported elsewhere by other researchers. We further demonstrate improved mixing over other cilia micromixers as enabled by the high aspect-ratio, high flexibility, and magnetic properties of our cilia micromixer elements.
Export citation and abstract BibTeX RIS
1. Introduction
Micromixers are essential components of labs-on-a-chip (LOC). Miniaturized systems designed for biological studies, analytical chemistry and life sciences frequently require micro-scale mixing for high sample throughput. However, fluid flow in microfluidic devices is typically laminar due to the low Reynolds numbers (usually <100) of microchannel fluid flow, resulting in little turbulence to aid in mixing of liquids [1]. With laminar flow, the slow molecular diffusion process limits the mixing efficiency. In microsystems with microchannel sizes above 1 µm, this effect is too slow to be used as the sole mixing mechanism [2]. Rapid mixing is crucial for many LOC devices and systems used in biological and biochemistry analysis such as enzyme reactions, RNA folding, protein folding and cell lysis [3, 4]. Development of effective micromixer structures that can be easily integrated with complicated microfluidic systems presents an important design challenge.
A number of microfluidic mixing methods have been demonstrated in the past two decades. Nguyen et al [4] and Capretto et al [2] give comprehensive reviews of different micromixer designs that have been proposed previously in the literature. Micromixers are generally classified as passive or active. Passive micromixers were the first mixers reported in the literature. Passive micromixing utilizes no external energy except for pumping the fluid inside the channels. In these devices, an enhancement in mixing is achieved by either generation of chaotic advection or the creation of heterogeneous surfaces to increase diffusion [1]. Passive micromixers often require less expensive fabrication techniques and are easier to integrate in complex micro total-analysis systems (µTAS) and LOC [2, 4] than active micromixers. However, passive micromixers often require extending the channel length, which is generally undesirable for miniaturization [1], and are usually optimized for one specific Reynolds number or for a specific fluid combination with specific rheological properties. Active mixers, on the other hand, use external energy to introduce dispersed multi-laminates or turmoil in the fluid that accelerates the mixing process [1, 2, 5]. As a result, active micromixing generally achieves higher mixing efficiency [6, 2]. For many applications, active mixers are also more desirable as they provide a more controllable mixing process via controlled stimuli. However, many active mixing systems have their own limitations. For instance, ultrasonic waves can produce high temperature gradients that can damage biological fluids [2, 4]. Therefore, an effective, non-damaging, and easy to implement active mixer design is still of high demand in the microfluidics field.
In order to address these problems, this paper proposes an active mixer based on controlled actuation of artificial cilia. Utilization of artificial cilia for mixing is a popular technique that has been relatively recently demonstrated in the literature [7–13, 15]. Various cilia actuation approaches have been demonstrated, such as magnetic force using large scale (20 × 3.5 × 2.5 mm) rotating permanent magnets connected to an electromotor [9], piezoelectric actuators to oscillate the cilia chamber [10, 11, 15], and electrostatically actuated cilia [8, 12]. Although each of these previously reported techniques enhance and accelerate the mixing process compared to diffusion-based passive mixing, they still have some limitations and disadvantages that are discussed in detail in section 5. In summary, some of these disadvantages include: complication in fabrication and integration with other polymeric microfluidic elements, difficult or impractical implementation of actuation techniques and low mixing efficiency.
In order to overcome many of these obstacles, high aspect-ratio structures with a low Young's modulus are required [11]. Such cilia should be fabricated using a simple fabrication technique compatible with microchannel and microchamber fabrication. Furthermore, such structures should be capable of a highly controllable and wide motion range employing relatively small stimuli that can be locally applied. This paper presents a new ultra-high aspect-ratio cilia micromixer, featuring rare-earth-doped polymer permanent-magnetic cilia to achieve efficient mixing. Rare-earth magnetic powder, (Nd0.7 Ce0.3)10.5 Fe83.9 B5.6, (MQFP-12-5 magnetic powder from Magnequench International Inc.), was used to dope polydimethylsiloxane (PDMS), resulting in a highly flexible magnetic nano-composite polymer (M-NCP) with the ability of providing bidirectional deflection when permanently magnetized [14, 16]. MQFP-12-5 powders are isotropic and can be magnetized equally well in any direction which eliminates consideration of magnetic alignment during the molding process [16]. This M-NCP was then used to create high aspect-ratio artificial cilia that can be actuated using miniature electromagnets to achieve a high range of vibration, creating turbulence inside the microfluidic reaction chamber. The application of such permanently magnetic polymer materials for fabrication of cilia structures has not been presented before in the literature, and presents a great advantage over cilia produced from iron-based and other soft-magnet ferromagnetic materials [9, 14] and mixers based on thin film magnets. In addition to demonstrating a new fabrication technique to realize the cilia, the mixing performance of mixers employing single cilium and arrays of multiple cilia were fully characterized and the results are presented in section 4. Approximately 85% of the total volume was fully mixed after 3.5 min using a single cilium mixer compared with only 20% of the total volume mixed with passive diffusion; this mixing performance is very promising and sufficient for our intended purposes for µTAS and LOC applications such as reagent mixing for polymerase chain reaction (PCR).
2. Design and fabrication
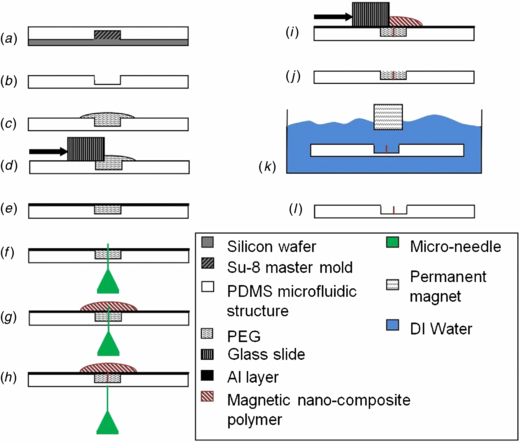
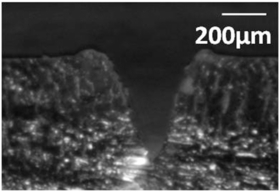
A technique has been developed for fabrication of artificial cilia directly into passive microfluidic structures, such as fluid microchannels (figure 1(a)) or reaction chambers (figure 1(b)). This technique is simple and can be easily scaled for volume production, and demonstrates that the cilia micromixers can be directly integrated with other microfluidic structures.
Figure 1. Artificial cilium structures fabricated directly inside (a) microfluidic channel (b) microfluidic reaction chamber.
Download figure:
Standard image High-resolution imageThe devices that were tested for mixing performance consisted of a reaction chamber where mixing between two liquids was performed. The fluid microchannels and chamber structures were fabricated in PDMS using standard soft-lithography techniques. The magnetic nano-composite polymer (M-NCP) was prepared by embedding magnetic powder in the PDMS polymer matrix. The magnetic powder used had a chemical composition of (Nd0.7Ce0.3)10.5Fe83.9B5.6, and contained a grain size in the range of 5–10 µm. The powder was uniformly dispersed with a weight percentage of 80 wt-% in the PDMS matrix using the same technique described in [17]. Figure 2 shows the mixer fabrication process. In order to fabricate the artificial cilia directly inside the reaction chamber, the chamber was filled with poly(ethylene glycol) (PEG) (average Mn = 2050 and melting point of 52–54 °C). PEG is a hydrophilic, biocompatible and water soluble polymer that has found widespread use in many biological applications, such as hydrogel fabrication. After being filled with melted PEG, the chamber was immediately covered with a thin sheet of aluminum (thickness <0.2 mm) in order to achieve a surface roughness of less than 15 µm, and allowed to cool down and solidify at room temperature for 10 min. In the next step, microneedles with diameters of 120 µm were inserted into the chamber (figure 2(f)). M-NCP was then poured over the microneedles and the needles were slowly pulled out. The vacuum created as the result of the evacuating microneedles drew the M-NCP into the holes, filling all voids. The sample was placed under vacuum for 30 min in order to ensure that there were no trapped air bubbles. Afterwards, the excess M-NCP on the surface was scraped off, the thin aluminum layer was peeled off, and the sample was allowed to cure at room temperature for 24 h. For the M-NCP preparation, the Dow Corning® 184 Silicone Elastomer kit was used, which has a working time of 1.4 h at 25 °C. This relatively short working time at room temperature (25 °C) allowed us to fully cure the M-NCP at room temperature within a reasonable time (24 h) without damaging the PEG mold. In the last step, the PEG mold was dissolved in 60 °C water. An external permanent magnet was used to keep the cilia in an upright position while they were taken out of the water and dried using a nitrogen gun, avoiding any collapse of the structures during drying.
Figure 2. Steps of the microfabrication process for M-NCP cilia. (a) Molding of PDMS. (b) Demolding of PDMS. (c) Filling chamber with PEG (Mn = 2050). (d) Removing excess PEG from the surface. (e) Covering with aluminum layer. (f) Inserting microneedle. (g) Pouring M-NCP. (h) Removing microneedle. (i) Removing excess polymer from the surface. (j) Curing. (k) Dissolving the PEG and (l) Releasing the cilia.
Download figure:
Standard image High-resolution imageIn order to make sure that the holes created on the base of the reaction chamber by the insertion of the microneedles were properly sealed by the M-NCP, leakage tests were performed. Figure 3 shows a schematic of the samples produced for the leakage test. In these samples, the inlet hole was connected to a Harvard Apparatus model 11 syringe pump through polyurethane tubes. The tube was fixed to the inlet hole using silicone sealant. The outlet hole was completely sealed using silicone sealant. The syringe contained dark blue dye to ensure fluid visibility in the channel. The pressure was gradually increased until a leakage was visually observed. In all the tested samples, there was no leakage under or around the microneedles' puncture points. Furthermore, leakage always first occurred either at the tube-inlet connection point or at lead-base junction between the two layers at around 1.5–2 psi. Therefore, the insertion of the microneedles in the chamber floor did not compromise the integrity of the reaction chamber because the insertion holes were fully sealed with the M-NCP.
Figure 3. Schematic of devices used for leakage tests: mixer/reaction chambers with single cilium mixer fabricated directly inside the chamber.
Download figure:
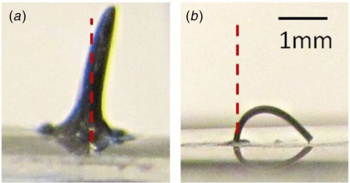
Standard image High-resolution imageUsing this simple fabrication technique, cilia structures exceeding an aspect-ratio of 8:0.13 were created. Fabrication of taller cilia fibers was not attempted because such high aspect-ratio fibers were not required for creating the micromixers which are the focus of this work. The maximum height for each cilium was limited by the reaction chamber height (1.7 ± 0.5 mm). The circular shape of the chamber, as well as the chamber dimensions of 4 mm in diameter and 1.7 mm in height, were chosen to ensure compatibility with: (1) commonly used microwell plates [11, 15] and (2) the multi-chamber PCR microfluidic system that is currently being developed at Simon Fraser University. Thus, each chamber was designed to contain approximately 20 µL of liquid. However, given the ease with which the cilia fibers were fabricated, aspect-ratios higher than 8:0.13 are expected to be achievable using this technique. Figures 4 and 5 show two examples of high aspect-ratio cilia microstructures with different aspect ratios.
Figure 4. High aspect-ratio cilium free-standing microstructure standing vertically in the absence of an external magnetic field. Cilium length(L) = 2.8 ± 0.01 mm and cilium diameter(D) = 130 ± 5 µm (average aspect-ratio of 2.8:0.13 = 21.54).
Download figure:
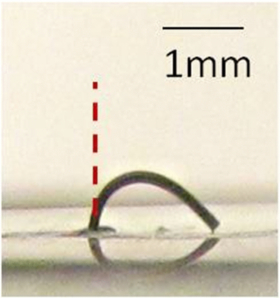
Standard image High-resolution imageFigure 5. Ultra-high aspect-ratio cilium fiber in the absence of an external magnetic field. L = 8 ± 0.1 mm and D = 130 ± 5 µm (average aspect-ratio of 8:0.13 = 61.54). Although such structures could be realized, they were not able to stand freely in the absence of an external magnetic field and were not employed as micromixer elements.
Download figure:
Standard image High-resolution imageExperimental tests results showed that cilia microstructures with a diameter of 130 ± 5 µm and a length of up to around 3.5 mm (average aspect-ratio of 3.5:0.13 = 26.92) are stiff enough to stand upright without any external magnetic field support, yet remain extremely flexible. As shown in figure 6, these cilia microstructures are capable of a full range of motion (90 degrees displacement of the cilium tip) under a relatively small magnetic field (110 mT). However, as the length of the cilia exceeded 3.5 mm, the cilia fibers started to bend under their own weight and external forces were required to keep them in an upright position; figure 5 shows an 8 mm long cilium bent under its own weight in the absence of a magnetic field.
Figure 6. Cilium structure in its full range of motion actuated using a small, 110 mT, permanent magnet. The cilium has diameter and length of D = 130 ± 5 µm and L = 2 ± 0.01 mm, respectively. In the absence of the external magnetic field, this cilium stands freely upright similarly to the microstructure shown in figure 4.
Download figure:
Standard image High-resolution imageCilia structures with different heights ranging from 500 µm to 2.8 mm and 130 ± 5 µm in diameter were employed for characterization tests. Thus, all cilia employed for testing were able to stand vertically on their own with no bending. The 120 µm diameter was the smallest diameter microneedle currently available to us for fabrication. This diameter is of sufficiently small size for micromixing in our reaction chambers (reaction chamber diameter = 4 ± 0.5 mm, reaction chamber height = 1.7 ± 0.5 mm) compatible with microfluidic systems and microwell plates [11, 15], and the PCR microchambers currently being developed at SFU, which requires the ability to mix a minimum reagent volume of 20 µL. However, considering the grain size of the magnetic powder used in this fabrication, 5–10 µm, it is expected that the minimum achievable feature size should be around 20 µm. Further investigation is required to confirm this estimation.
In order to also characterize the effect on mixing performance of multiple artificial cilia, differently sized arrays of multiple cilia were fabricated inside the reaction chamber. Figure 7 shows the arrangement of different numbers of cilia in each mixing chamber. The fabrication technique for these devices is the same as the procedure explained in figure 1; the only difference is that in this case multiple microneedles must be inserted in different locations inside the chamber. For the results presented in this paper, the needles were inserted by hand and individually. However, for high throughput and large scale fabrication process, customized stamps could be created and controlled by robotic arm for higher placement precision and faster microfabrication processing. While 1 mm distances were used between cilia for demonstration purposes, using our technique, we are able to place cilia much closer together, within 250 ± 50 µm of each other. This distance could be further reduced if needed by fabrication of high precision stamps or using a robotic arm for placement of the individual microneedles.
Figure 7. Cilia arrangement in the mixing chamber for multiple cilia mixers. These illustrations are from a viewpoint looking down into the chamber and showing the placement of the multiple free-standing vertical cilia. The distance between each two adjacent cilium is approximately 1 mm. This diagram is for illustration purpose and does not represent exact dimensional ratios.
Download figure:
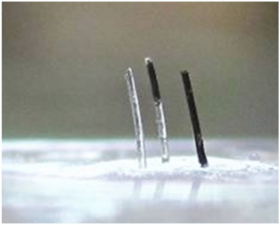
Standard image High-resolution imageCilia in which only part of the cilia lengths were doped with magnetic particles were also made to demonstrate the versatility of the proposed fabrication technique. Previous research has shown that doped PDMS has a higher Young's modulus and hence lower flexibility compared with un-doped PDMS [14]. Therefore, in applications where maximum flexibility is required, it can be beneficial to be able to fabricate partially doped cilia structures. In order to achieve optimal flexibility based on the application requirements, any combination of partially doped cilia can be fabricated on the same microfluidic chip using the technique suggested in this paper (figure 8).
Figure 8. Fabricated cilia array with different ratio of doped to un-doped PDMS sections along the cilia lengths. 0% of the length doped on the left, 40% of the length doped in the middle and 100% of the length doped on the right (doping level at 80 wt-% magnetic particles in the PDMS matrix).
Download figure:
Standard image High-resolution imageThe most direct comparison between the cilia structures presented in this paper and other cilia mixer elements presented in the literature may be made with the mixer cilia of Riahi et al [9], since the geometrical design and structural resemblance between the two proposed mixer elements (cilia) are the closest. In [9], Riahi et al used a CO2 laser machine to make cone shape holes on PMMA, which were then used as cilia molds. The holes were first filled with ferromagnetic iron (Fe) microparticles and then covered with PDMS to form Fe-doped cones which were then placed in PMMA microchannels to act as individual mixer elements. In order to directly compare the mechanical characteristic of the cone shaped structures suggested by [9] with the cylindrical structures suggested in the present work, similar structures using the same method suggested by Riahi at al were fabricated and compared to the cilia structures made using the technique suggested in this paper. A Universal Laser System's VersalLASER© Laser ablation system was used to pattern the PMMA mold. The holes were then filled with (Nd0.7Ce0.3)10.5Fe83.9B5.6 M-NCP, a material with much higher magnetization than the weakly magnetic Fe-doped polymer [16] suggested by Riahi et al [9]. Channels and holes fabricated using the CO2 laser cutter exhibited a Gaussian profile (figure 9). Using these structures as the master mold for fabrication of PDMS cilia resulted in cone shaped pillars (figure 10(a)). It proved very difficult to precisely control the diameter and the depth of the holes. The cone shape structures had significantly lower flexibility when compared to perfectly cylindrical shaped cilia made using the new fabrication technique presented in this paper (figure 10(b)). Figure 10 shows two cilia structures with almost the same height realized using the two different fabrication techniques. The structure made using a laser cutter resulted in an aspect-ratio of 2:0.7. The structure made using the technique described in this paper has an aspect-ratio of 2:0.13. In figure 10, both of the structures were actuated using an 110 mT permanent magnet. It is clear that, while both of the cilia are made of PDMS and doped with the same M-NCP, the cylindrical cilium shows a very high degree of deflection whereas there is much less deflection for the cilium made using the laser cutter-based molds.
Figure 9. Cross section of a microchannel created using Universal Laser System's VersalLASER© Laser ablation system CO2 laser.
Download figure:
Standard image High-resolution imageFigure 10. Cilia structures fabricated from the same material using two different techniques. (a) Using a mold made by patterning commercial poly(methyl methacrylate), PMMA, using a CO2 laser cutter (height = 2 ± 0.5 mm), similarly to [9] except employing highly magnetic rare-earth materials and (b) using the new fabrication technique described in this paper (height = 1.8 ± 0.01 mm). Both of these structures were actuated using an 110 mT permanent magnet, showing higher flexibility for cilia fabricated using the new fabrication technique presented in this paper.
Download figure:
Standard image High-resolution imageAs a result of the low magnetization of the Fe-doped polymer and the reduced flexibility due to the cone shape of the cilium, Riahi et al needed to put a large permanent magnet (length = 20 mm, width = 3.5 mm and height = 2.5 mm) providing 75 mT next to the cilia in order to be able to deflect the beam sufficiently (illustrated in figure 11 (left)). Having a large scale magnet connected to an electromotor next to each mixer microstructure adds considerable complexity to the packaging of the microfluidic chip and is impractical for complicated microfluidic systems with multiple chambers and mixers. However, in the technique proposed in this paper, sufficient deflection can be achieved using miniature electromagnets providing 7 mT. Each electromagnet can be located underneath or above the chamber, (illustrated in figure 11 (right)), which is far more manageable for large scale or complex multi-chamber/multi-mixer chip designs.
Figure 11. (Left) Actuation mechanism suggested by Riahi et al [9]. (Right) actuation mechanism suggested in this paper. The rotating magnet on the left needs to be orders of magnitude stronger than the electromagnet used in the right-hand diagram due to the lower aspect-ratio of the structure, and hence lower deflection range for a given magnetic field.
Download figure:
Standard image High-resolution image3. Experimental apparatus
Two complete sets of characterization experiments were carried out to determine the mixing performance and efficiency of the cilia micromixers. In the first set of characterization tests, the effects of different cilia lengths, excitation frequencies, and excitation amplitudes on mixing performance of a single cilium microstructure were studied. In the second set of tests, the effect of arrays of multiple cilia on the mixing performance was studied. In all of these tests, miniature electromagnets (tubular electromagnets from Magnetic Sensor Systems series E-66-38) were used to locally actuate the cilia (figure 12).
Figure 12. Miniature electromagnet employed for cilia microactuation. Outer cylinder: diameter = 9.6 mm, height = 16.7 mm.
Download figure:
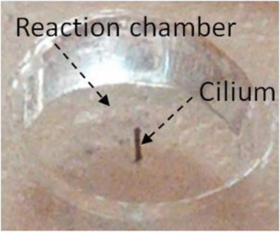
Standard image High-resolution imageThe electromagnets were first powered by a signal generator that provided square waves with frequencies ranging from dc to 120 Hz at 20 V. To test the effect of different actuation amplitudes on the mixing performance, the electromagnets were then powered with different voltages ranging from 0 to 20 V at 60 Hz, which corresponded to the natural frequency of the 1.5 mm tall cilium used in these sets of tests. A microscope mounted CCD camera was used to capture videos that were later converted into image sequences, which were analyzed to obtain the results shown in the section 4. We realize that vertical mixing may be an issue, as our results are shown using two-dimensional microscopy. We plan to explore this aspect of the mixing performance in a future work. Cilia microstructures with a height of 1.5 mm were fabricated in the bottom of a reaction chambers (figure 13). The reaction chamber had a diameter of 4 mm and a height of 1.7 mm, large enough to hold approximately 21 µL of fluid. In addition, circular shape reaction chambers were chosen to demonstrate compatibility with microwell plates. It should be noted that much smaller microchambers or smaller microchannels could also be employed using the same fabrication process.
Figure 13. Testing reaction chamber used to capture cilia mixer performance. This example chamber has a diameter = 4 ± 0.5 mm and a height = 1.7 ± 0.5 mm. Cilium diameter = 130 ± 5 µm, cilium length = 1.5 ± 0.01 mm.
Download figure:
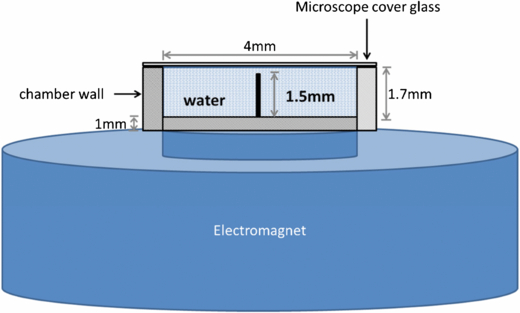
Standard image High-resolution imageLiquids were directly inserted into the chamber using Hamilton microsyringes. The chambers were then covered with a thin microscope cover glass to prevent evaporation of the samples and maintain surface transparency to ensure good image quality for analysis. The miniature electromagnet was directly located under the mixing chamber touching the bottom of the chamber (figure 14). A volume of 0.1 µL of red and green dye was mixed with 20 µL of DI-water to allow for optical analysis of the mixing process (figure 15).
Figure 14. Cross sectional view of the configuration of the mixing chamber and electromagnet employed for testing. This diagram is for illustration purpose and does not represent exact dimensional ratios.
Download figure:
Standard image High-resolution imageFigure 15. Sample images of the mixing process compared to diffusion alone. Plots (a) and (b) show 60 Hz actuation of the cilium at times t = 0 min and t = 15 min, respectively. Plots (c) and (d) show natural diffusion at times t = 0 min and t = 15 min, respectively, with no cilium actuation.
Download figure:
Standard image High-resolution image4. Experimental results
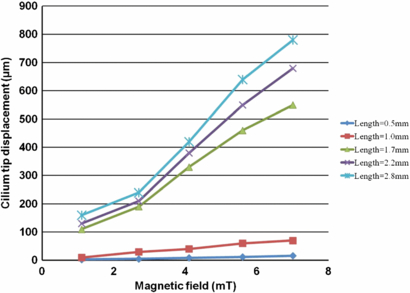
Cilia structures 130 ± 5 µm in diameter and ranging in height from 500 µm to 2.8 mm were actuated in electromagnetic fields ranging from 1.1 to 7 mT. As previously mentioned, miniature electromagnets, such as that shown in figure 12, were employed to actuate the cilia. Table 1 shows the relationship between voltages applied to the electromagnets, generated magnetic field as measured by an F.W.Bell® Hall effect Gauss/Tesla meter model 5180, and resulting cilium displacement for different lengths. This data shows that, for a fixed magnetic field and frequency, the taller a cilium structures is, the larger the cilium vibration amplitude will be and hence the better its mixing performance. Therefore, for a given size reaction chamber, it is best to have the tallest possible cilium mixer that can be fit into the chamber and can freely vibrate without touching the top surface. This is approximately a few hundreds of micrometers shorter than the chamber size for our cilia size ranges to insure the free movement of the cilia inside the chamber.
Table 1. The relationship between voltages applied to the electromagnet, generated magnetic field as measured by Tesla meter, and resulting cilium tip displacement. All of these structures were actuated using 60 Hz square waves and all the cilia structures had a diameter of 130 ± 5 µm.
| Cilium length | ||||||
|---|---|---|---|---|---|---|
| 500 µm | 1 mm | 1.7 mm | 2.2 mm | 2.8 mm | ||
| Applied voltage (V) | Resulting magnetic field (mT) | Average Cilium tip's displacement (µm)a | ||||
| 2 | 1.1 | 5 | 10 | 110 | 130 | 160 |
| 4 | 2.7 | 5 | 30 | 190 | 210 | 240 |
| 6 | 4.1 | 9 | 40 | 330 | 380 | 420 |
| 8 | 5.6 | 12 | 60 | 460 | 550 | 640 |
| 10 | 7 | 16 | 70 | 550 | 680 | 780 |
As shown in figure 16, for a given cilium length, as the applied magnetic field increases, the displacement increases and this increase is more profound for longer cilia.
Figure 16. Relationship between the cilium length, applied magnetic field and cilium tip displacement. Cilium diameter = 130 ± 5 µm. Actuation frequency = 60 Hz. All the displacement values have measurement accuracy of ± 2 µm.
Download figure:
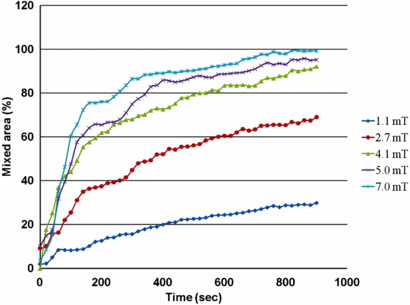
Standard image High-resolution imageFigure 17 shows the mixing efficiency of a single cilium micromixer at various frequencies and using a 7 mT magnetic field. As can be seen in this figure, the 0 Hz actuation (passive mixing by natural diffusion) results in a very small partially mixed area inside the chamber by the natural diffusion process. Only approximately 20% of the chamber's total volume was partially mixed after 15 min using such diffusional mixing alone. However, in the case of active mixing (10–120 Hz) more than 85% of the volume was fully mixed after approximately 3.5 min and almost 98% mixing was achieved after about 10 min. Table 2 shows that the cilium micromixer reduced mixing time by more than 16 times over passive diffusion.
Figure 17. Experimental cilium mixing results showing percentage mixed over elapsed time for different actuation frequencies at 7 mT. 0 Hz is no actuation (diffusion only). Cilium length = 1.5 ± 0.01 mm, cilium diameter = 130 ± 5 µm.
Download figure:
Standard image High-resolution imageTable 2. Mixing efficiency of single cilium micromixer compared to natural diffusion.
| Mixing method | Approximate time to reach 20% of the total volume mixed (s) |
|---|---|
| Natural diffusion (0 Hz) | 800 |
| Cilium mixing (10–120 Hz) | 50 |
Figure 18 shows the effect of different applied magnetic field strengths on the mixing performance of the cilium microstructure at 60 Hz. As the magnetic field increases, the mixing performance of the cilium improves due to the increase in the cilium vibration range resulting from the higher applied magnetic field. Also, figures 17 and 18 show that increasing the vibration amplitude of the cilium has a more profound effect on mixing performance than increased frequency.
Figure 18. Experimental cilium mixing results showing percentage mixed over elapsed time for different applied electromagnetic fields. Cilium length = 1.5 ± 0.01 mm, cilium diameter = 130 ± 5 µm.
Download figure:
Standard image High-resolution imageThe effect of increasing the number of cilia per chamber was next studied. Figure 19 shows an example of a 2 × 3 array of cilia micromixer elements with a roughly 1 mm gap between the cilia.
Figure 19. A 2 × 3 array of six cilia. Cilium diameter = 130 ± 5 µm, cilium height = 1.5 mm, gap between the cilia = 1 ± 0.05 mm.
Download figure:
Standard image High-resolution imageIt was discovered experimentally that, for cilia taller than 1 mm, as the gap size decreases below 500 ± 50 µm, the attraction between highly magnetized M-NCP cilia structures causes them to bend towards each other and sometimes collapse into one another. For 0.5 mm tall cilia, the distance between cilia can be decreased to around 200 µm. The probability of collapses increases as the cilium height increases.
While magnetizing the cilia limits the spacing, this approach offers two distinct advantages. First, the addition of permanently magnetic particles to the cilia allows for bidirectional actuation. Non-magnetized cilia will only attract to the actuation field, while magnetized cilia will alternatively attract and repel. This results in actuation at a lower frequency with greater displacement. Second, compared to previous designs that were doped with weakly magnetic particles (e.g. Fe-based), the proposed design using highly-magnetic rare-earth particles exhibiting much higher displacement for the same actuation magnetic field. Permanently magnetized MQFP-12-5 offer both a higher magnetic saturation level and a magnetic remanence which is four times that of isotropic ferrite powders and two times of anisotropic ferrite powders [16]. Furthermore, with ultra-high aspect-ratio structures such as cilia, spatial densities beyond this point may result in physical interactions between the vibrating cilia, putting another physical limitation on the displacement. The proposed design prioritizes maximizing the displacement of the cilia, which has the biggest impact on the effectiveness of the mixer (as shown in figures 17 and 18). Future characterization work will focus on optimizing the level of magnetization with the density of cilia structures to produce maximum mixing efficiency.
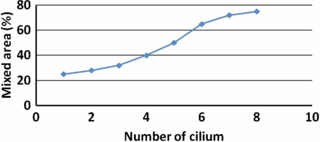
Figure 7 shows the arrangement of the different sizes of cilia in the micromixer chambers used for the characterization of multi-cilia micromixers. Similar to the previous tests, 1.5 mm tall cilia structures with diameters of 130 ± 5 µm were fabricated in chambers with a diameter of 4 mm and a height of 1.7 mm. As shown in figure 20, increasing the number of cilia in a chamber increases the mixing efficiency significantly, with eight cilia mixing approximately twice the volume as four cilia after the same time period of 1 min with the same magnetic field strength (7 mT) and frequency (60 Hz). Further characterization is required to achieve an optimal design with regards to the number of cilium per reaction chamber and cilium height and spacing to further improve the mixers performance. Such optimization will be carried in future work.
Figure 20. Mixer performance for different number of cilia microstructures per chamber after 1 min of mixing at 60 Hz and applied magnetic field of 7 mT.
Download figure:
Standard image High-resolution image5. Discussion
Fabrication of successful cilia structures for mixing purposes has been previously reported by other researchers [7–13]. However, in most cases there were factors that limited the practicality or the performance of the suggested cilia micromixers in LOC and µTAS applications. In this section, some of these limitations are described and it is shown how the new designs suggested in this paper may eliminate or reduce the impact of some of these limitations on fabrication and/or performance of cilia-based micromixers.
Timonen et al [7] presented flexible magnetic artificial cilia based on spontaneous surface deformation of ferrofluids in the presence of a magnetic field. Cobalt particles were mixed with toluene and an elastomeric polymer. In the presence of the magnetic field, the cobalt particles formed conical structures. While still under an applied magnetic field, the toluene was allowed to evaporate and the polymer solidified. The length and the aspect-ratio of the cilia structures depended strongly on the vertical magnetic field gradient applied during the cilia formation. Aspect-ratios of 20 and 120 were achieved under 0.1 and 20 T m−1 vertical gradients, respectively. Their presented technique has many positive aspects, such as mechanically stable structures, ease of fabrication and actuation using moderate magnetic fields of 450 mT. However, in the fabrication technique suggested by Timonen et al, it was relatively hard to precisely control the density, length, diameter and thickness of the cilia fibers. As the suggested technique resulted in a high density of imprecisely placed cilia, the cilia could potentially act as particle traps. Hence, the structures reported by Timonen et al may not be suitable for use as a mixer for liquids that contain particulates or biological entities of sufficient size to become trapped. In contrast, the technique presented in this paper results in precise positioning of each individual cilium. The length, height and diameter of each cilium can also be individually determined and placed precisely. Figure 21 shows an array of four cilia with four different lengths ranging from 0.8 ± 0.05 to 2.2 ± 0.05 mm, each with a diameter of 130 ± 5 µm, made on the same sample and actuated using a 7 mT miniature electromagnet at 60 Hz. A longer exposure was used while taking the image in order to show the vibration range in a single image and the control that the new process has in terms of individual cilium placement and size. In addition, Timonen et al found that if the magnetic field was suddenly removed, their cilia structures never completely relaxed back to a vertical position without applying a vertical magnetic field to erect the cilia. With the design presented in this paper, whenever the magnetic field is removed, the cilia relax to their original well-controlled positions. For example, in one experiment, it took less than 0.5 s for the cilia structures to relax back to their original vertical position after removal of the magnetic field. Similarly to Riahi et al [9] discussed in section 2, Timonen et al [7] also does not discuss the performance of micromixers employing their cilia. Thus, it is not possible to compare the mixing performance of the mixers presented in this paper with either of those proposed in [7] or [9].
Figure 21. An array consisting of four cilia microstructures with different sizes actuated using a 7 mT electromagnet at 60 Hz (cilium height: top left = 2.2 ± 0.05 mm, bottom left = 2 ± 0.05 mm, bottom right = 1.6 ± 0.05 mm, and top right = 0.8 ± 0.05 mm; the cilium diameter = 130 ± 5 µm for all four cilia). This demonstrates the precise control over cilia height, placement, and actuated tip displacement possible with the new fabrication method.
Download figure:
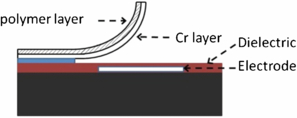
Standard image High-resolution imageThe cilia structures demonstrated by den Toonder et al [8, 12] are based on electrostatically actuated polymer structures. The den Toonder et al cilia structures were curled microbeams consisting of a double layer of a thin polymer film, polyimide, and a thin conductive chromium layer. The cilia had a length of 100 µm and a width of 20 µm, and were actuated by applying a voltage difference between the indium tin oxide electrode and the Cr layer (figure 22).
Figure 22. Cross sectional view of the cilia structure reported in [8].
Download figure:
Standard image High-resolution imageBecause of the relatively small size of their structures, the structures presented by den Toonder et al may be advantageous over the design presented in this work for microfluidic systems that require microchannels with very small sizes (e.g., <100 µm). However, the den Toonder et al mixer suffered from problems of fabrication complexity and durability. As reported in [8], these mixers showed signs of failure after 600 000 switching cycles at 1 Hz [8]. As well, the mixers demonstrated by den Toonder et al required a relatively complicated multilayer fabrication process with access to more expensive microfabrication equipment such as a sputtering machine and plasma enhanced chemical vapor deposition (PECVD). The relatively complex fabrication techniques are a limiting factor for rapid prototyping applications or where low fabrication cost is required. In contrast, the fabrication technique described in this paper is simple and of relatively low cost. Furthermore, cilia structures made using the PDMS-based microfabrication described in this paper are flexible and are not prone to fracture due to mechanical stress or handling of the microfluidic chip. For example, there was no sign of any failure or fatigue after running the M-NCP cilia mixers for 168 h, one week, at 60 Hz either with or without liquid, which represents approximately 36 000 000 cycles. From a mixing performance point of view, the performance of an array of cilia structures suggested by [8] is comparable with the performance of a single cilium mixer suggested in this paper. The single cilium mixer presented in this work results in a mixing time per volume of liquid (T90%mix/V) of 10 s µL−1 and this value reduces to less than 2.7 s µL−1 for an array of eight cilia. This is similar to T90%mix /V ranging from 10–1 s µL−1 reported by den Toonder et al [8].
Oh et al [10, 11, 15] have presented a mixer that consists of PDMS cilia structures actuated by the vibration of an off-the-shelf lead-zirconate-titanate (PZT) piezoelectric stage. Cilia structures as tall as 400 µm were fabricated using a novel underwater fabrication technique. The cilia structures were also fabricated separately and then assembled horizontally inside the chambers, which required additional assembly steps. As all cilia must be actuated at once by the piezoelectric stage used for cilia excitation, the mixers described by Oh et al are limited in that multiple micromixers on the same chip cannot be individually, locally controlled, and all mixing chamber must be actuated at once with the same parameters. The technique described by Oh et al is particularly limiting if there is a need to have independent control over individual chambers' excitation frequency and/or amplitude, or even whether fluid in each chamber is being actively mixed or not. The actuation technique described by Oh et al also requires complicated packaging, especially as the number of mixing chambers and the complexity of the design increases. In contrast, the fabrication process employed for the micromixers in this work allows production of the cilia structures directly inside the microfluidic system with no additional assembly steps. By using miniature electromagnets for each micromixer, each mixing chamber can be controlled individually. Comparison of the mixing performance of the new micromixer structures suggested in this work and the mixer presented by Oh et al is shown in table 3.
Table 3. Characteristic parameters of micromixers for Oh et al [11, 15] and the present work.
| Diffusion | Cilia mixer | ||||||
|---|---|---|---|---|---|---|---|
| Reference | Cilium per chamber | Volume (µL) | Approx. T90%mix (s) | T90%mix/V (s µL−1) | Approx. T90%mix (s) | T90%mix/V (s µL−1) | [T90%mix/V]diff/[T90%mix/V]mix |
| Oh et al | 3 | 7 | 651 | 93 | 81 | 11.6 | 8 |
| The present work | 3 | 20 | 2700 | 135 | 120 | 6 | 122.5 |
6. Conclusion
In this paper, a new micromixer is realized employing a new fabrication technique, resulting in ultra-high aspect-ratio cilia microstructures. The cilia microstructures fabricated using this new technique are used as active mixers. The mixing performance of these cilia mixers are experimentally characterized in comparison with passive diffusion-based mixers. The experimental result shows that a single cilium structure reduces the 90% mixing time per unit volume by about 122 times compared to passive diffusion ([T90%mix/V]diff/[T90%mix/V]mix = 122.5). The performance of the mixer while actuated under different frequencies and different magnetic fields is evaluated. It is shown that the change in the actuation magnetic field has a more profound effect on the mixing efficiency than the change in frequency. Increasing the amplitude of the magnetic field results in an increase in the vibration range of the cilia and hence increases the amount of turbulence created enabling higher mixing efficiency. Using an array of cilia structures, instead of a single cilium mixer, reduces this mixing time even further. Approximately 85% mixing can be achieved in about 70 s by using an array of eight cilia, as opposed to 3.5 min for single cilium mixer, when both are actuated under the same 7 mT and 60 Hz frequency magnetic field. The new fabrication technique proposed in this work is relatively easy as well as being low cost. It can be used for high volume and large scale productions. Using highly magnetized and highly flexible M-NCP cilia resulted in creation of very responsive actuators that can be actuated using a relatively small and localized magnetic field, 7 mT, created by miniature electro magnet. The combination of effective fabrication, actuation technique, and effective mixing performance of these cilia micromixers make their design attractive for integration with many complex microfluidic designs in LOC and µTAS devices.
Acknowledgments
This research was supported by Canadian National Engineering and Science Research Council (NSERC) for funding our research, as well as the Canadian Foundation for Innovation for equipment. We also would like to thank our collaborators at Neomaterials and Magnequench International for supplying in-kind contributions of rare-earth magnetic Magnequench fine powders. Finally, we would like to extend our thanks to Dr Ajit Khosla for his supports and great contributions to the Microinstrumentation Laboratory magnetics materials research, and thank Hsiu Yang Tseng for his great input with the testing apparatus and Nicholas C Doyle for reviewing the manuscript.