Abstract
Uncertainties in determining clinically used relative biological effectiveness (RBE) values for ion beam therapy carry the risk of absolute and relative misestimations of RBE-weighted doses for clinical scenarios. This study assesses the consequences of hypothetical misestimations of input parameters to the RBE modelling for carbon ion treatment plans by a variational approach. The impact of the variations on resulting cell survival and RBE values is evaluated as a function of the remaining ion range. In addition, the sensitivity to misestimations in RBE modelling is compared for single fields and two opposed fields using differing optimization criteria. It is demonstrated for single treatment fields that moderate variations (up to ±50%) of representative nominal input parameters for four tumours result mainly in a misestimation of the RBE-weighted dose in the planning target volume (PTV) by a constant factor and only smaller RBE-weighted dose gradients. Ensuring a more uniform radiation quality in the PTV eases the clinical importance of uncertainties in the radiobiological treatment parameters, as for such a condition uncertainties tend to result only in a systematic misestimation of RBE-weighted dose in the PTV by a constant factor. Two opposed carbon ion fields with a constant RBE in the PTV are found to result in rather robust conditions. Treatments using two ion species may be used to achieve a constant RBE in the PTV irrespective of the size and depth of the spread-out Bragg peak.
Export citation and abstract BibTeX RIS
For more information on this article, see medicalphysicsweb.org
1. Introduction
It is current clinical practice in carbon ion radiotherapy (RT) to take the variation of the beam quality of the radiation field with depth into account in order to deliver an isoeffective dose to the tumour in the planning target volume (PTV) (Krämer and Scholz 2000). Uncertainties in predicting relative biological effectiveness (RBE) values for tumours and tissues for a given treatment can lead to a misestimation of RBE-weighted doses, in the following referred to as biological dose, and may result consequently in a sub-optimal treatment. The cell survival and RBE values of tumour cells and normal tissues depend on a variety of factors including, amongst others, notably the biological end-point, the cell-phenotype, individual genetic parameters and specific mutations, nutrient supply and the state of oxygenation during the treatment. The uncertainty of isoeffective dose for carbon ion treatments is estimated to be about 20% (Karger and Jäkel 2007). In the past, the evolution of biophysical models used for treatment planning showed variations of predicted biological doses (Elsässer et al 2008b) which led to the retrospective evaluation of treatment plans (Gillmann et al 2011). There are differing sources of uncertainties for the biophysical models and parameters which are used to establish the clinically used RBE values. Currently, the radiosensitivity of the treated tumour (and normal tissue) in a clinical setting can generally only be predicted with limited accuracy and is not patient-specific. Consequently, it is common clinical practice for ion therapy to model different types of tumours and normal tissues for treatment planning by a single biological system. However, a large variability in tumour radiosensitivity can be expected between patients and even within a given tumour. Until now, the most relevant clinical experience using scanned carbon ion beams in terms of number of treated patients and length of the follow-up was obtained by using RBE values predicted by the first version of the local effect model (LEM), referred to as LEM-I, for chordoma (Krämer and Scholz 2000, Jäkel et al 2001). At NIRS (National Institute of Radiological Science, Japan), clinical experience for scattered carbon ion beams is largely based on the use of data from human salivary gland (HSG) cells for modelling tumours and normal tissues together with clinical data gained from neutrons (Kanai et al 1999, 2006). Recently, NIRS has also started treatment with scanned carbon ion beams (Kamada 2012). Some prior investigations of the accuracy of RBE values for ion beam therapy have been undertaken which focus mostly on the accuracy of experimental data and biophysical models (Elsässer et al 2008a, 2008b, Friedrich et al 2009, 2010) without examining directly consequences for treatment plans. Other studies investigated the impact of using a variable RBE for proton treatment plans (Tilly et al 2005, Carabe et al 2012).
This study assesses the consequences of hypothetical misestimations of input parameters to the RBE modelling for carbon ion treatment plans by a variational approach using a re-implementation of the LEM model. The method allows one to estimate the relative sensitivity to changes in input parameters for the biophysical modelling and evaluates the severity of the impact of their misestimation in terms of biological dose distributions and cell survival. Biological parameters describing the radiosensitivity to photon irradiation, i.e. αx and βx, as well as LEM-intrinsic model parameters are varied. Using the LEM, variations of radiosensitivity to photon irradiation are translated consistently to variations for ions. This allows also for a relative comparison of resulting uncertainties for the two treatment modalities. Moreover, differences in RBE predictions for different tumours and LEM versions are examined. The robustness of carbon ion treatment fields to changes in biological parameters is evaluated for various types of treatment plans, including carbon ion treatments in water phantoms with single fields and opposed fields using different optimization criteria. In addition, the effect of the size and depth of the spread-out Bragg peak (SOBP) on possible misestimations of RBE values is assessed. Radiations of different quality result in different biological and clinical effects for the same absorbed dose. Differences in radiation quality are related to differences in the microscopic energy deposition patterns. It is demonstrated how a more uniform radiation quality in the PTV can be used in order to reduce the clinical impact of misestimations of the RBE values, easing thereby the required accuracy for the biological modelling and its input parameters.
Treatment planning is carried out in the current study using a newly developed Monte Carlo-based treatment planning (MCTP) tool. Cell survival and RBE values are predicted with the HIT (Heidelberger Ionenstrahl-Therapiezentrum, Germany) re-implementation of the LEM in the flavour of LEM-IV (Elsässer et al 2010). The LEM-IV is a successor of the LEM-I, which is currently the standard model clinically used for predicting RBE values for scanned ion beam therapy.
In this paper, section 2 discusses general aspects of uncertainties of biological treatment parameters for ion RT and defines different types of uncertainties for treatment planning. Details about the biological modelling, variations of the modelling input parameters, the treatment planning tool and optimization approaches are given in section 3. Section 4.1 presents a sensitivity study for LEM input parameters in terms of resulting RBE and cell survival variations. Section 4.2 investigates the impact of uncertainties in the biological modelling for single-field and opposed-field treatments in water phantoms using differing optimization approaches. Finally, section 4.3 discusses some assumptions of the presented study and adds general remarks.
2. Aspects of uncertainties in biological treatment parameters for ion beam therapy
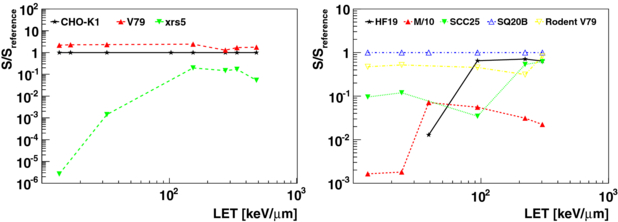
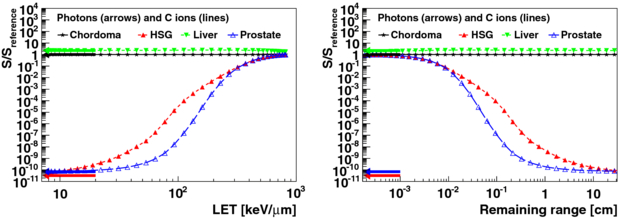
When compared to sparsely ionizing photon radiation, densely ionizing ion radiation causes generally more complex and severe damages to cells which are difficult to repair. Consequently, the impact of damage by densely ionizing radiation, such as ions with a high linear energy transfer (LET), in terms of cell survival is found to be less dependent on alterations, such as mutations affecting repair capabilities, and conditions of the tumour and normal tissue cells, such as hypoxia. Latter insensitivity is expressed by a lower oxygen enhancement ratio (OER) which is experimentally found for densely ionizing radiation (e.g. Barendsen et al 1966, Furusawa et al 2000). The trend for the decreased variation of cellular response to densely ionizing irradiation is one of the assets for carbon ion therapy. Figure 1 illustrates this trend for carbon ion radiation for various cell lines for the same absorbed dose. Doses that yield a survival of 10% for reference cell lines (CHO-K1 and SQ20B) are shown together with corresponding survival of other cell lines for the same doses. Cell survival parametrizations of data from clonogenic assays are taken from Weyrather et al (1999) and Belli et al (2008). They include three types of hamster cell lines: V79, CHO-K1 and the radiosensitive CHO mutant xrs5 and four human normal and tumour cell lines: HF19, M/10, SCC25, SQ20B together with rodent V79. The decreased spread of biological response for densely ionizing radiation implies that by relating the biological effect of high-LET ions to the effect of sparsely ionizing photons, one tries to describe a biologically more stable effect based on a quantity which is subject to much larger variations due to individual cell and tissue characteristics, such as the cell repair capacities and its oxygenation state. Experimental data from different cell lines (e.g. Belli et al 2008, Furusawa et al 2000, Weyrather et al 1999) as well as some scarce in vivo data (e.g. Ando et al 1998, 2006, Battermann et al 1981, Karger et al 2006) are generally used for developing, matching and benchmarking biophysical models which are aimed to predict clinical effects of different ion radiation qualities. However, the knowledge about cell survival and clinical end-points for photons, such as tumour control and normal tissue complications, is significantly more advanced. Presently, there exists only a relatively scarce data pool for high-LET ion irradiation compared to photons. Therefore, it is an important cornerstone of the current strategy for carbon ion therapy to exploit and translate knowledge gained in RT with photons (and with neutrons) in order to apply it to ion RT (Linz et al 2012). This implies that for ion RT, additional uncertainties due to the translation process add to the uncertainties from photon and neutron data. Uncertainties in clinical RBE values used for ion RT may be divided into four categories, according to their origin.
- (i)Uncertainties in the experimental data, such as cell survival and tissue response, used to validate mathematical RBE modelling and to establish clinical RBE values. For instance, for cell survival assays and animal experiments factors such as statistical uncertainties, dosimetric aspects and uncertainties linked to the studied biological system may play a role.
- (ii)Uncertainties in the biophysical models that are used to predict RBE values based on (i) for treatment planning. This includes the following.
- Possible systematic false predictions introduced by the model formalism itself.
- Uncertainties in the model input parameters which often reflect uncertainties in the underlying experimental data and radiobiological models used for determining these parameters.
- Uncertainties due to the applied fitting procedures and due to inter- and extrapolation by the model.
- (iii)Uncertainties arising from the fact that the biological system that is modelled (ii) is not necessarily the one that is treated in clinical practice. This comprises many aspects, amongst others notably the following ones.
- Inter-tumour variability and inter-patient variability of tissues, for instance due to genetic differences and mutations.
- Local variability and inter- and intra-fraction variability, e.g., due to acute and chronic hypoxia, nutrition supply, cell-cycle effects, etc.
- General differences for in vitro data, which are often used for model verification, and in vivo data.
- (iv)Non-biological, but mostly physics-related uncertainties for the treatment. For instance, uncertainties in the radiation field such as its fragment fluence composition.
Figure 1. Doses that yield a survival of Sreference = 10% for the reference cell lines: CHO-K1 (left) and SQ20B (right) are shown together with corresponding survival S of other cell lines for the same doses. Cell survival parametrizations of data from clonogenic assays are taken from Weyrather et al (1999) (left) and Belli et al (2008) (right).
Download figure:
Standard imageWith respect to their clinical relevance, it is important to discriminate between different types of possible misestimations of biological effects. Uncertainties resulting in a systematic but constant mean under- and over-dosage of the tumour volume and normal tissues for a given treatment protocol, referred to as absolute misestimation, can be detected by clinical dose escalation trials and can be corrected adapting the prescribed biological dose to the PTV by finding a compromise between tumour control probability (TCP) and normal tissue complication probability (NTCP). It is clinical practice in carbon ion therapy to evaluate the adequate biological dose for a given treatment protocol by clinical dose escalation studies (Linz et al 2012, chapter 30, p 517). In contrast, uncertainties in the prediction of the biological doses which lead to biological dose gradients in the tumour volume or a misestimation of biological dose as a function of treatment parameters, such as size and depth of the SOBP, can be assumed to result generally in a worse treatment outcome10 by either decreasing TCP or increasing NTCP. Such relative systematic mismatches, referred to in the following as relative misestimations, are by far more difficult to detect clinically and result generally in a flattened dose-response relationship. In contrast to photons (and also neutrons (Wambersie and Menzel 1997)), which have in good approximation a uniform radiation quality in the treated volume, therapeutic ions exhibit significant changes of radiation quality and add an additional dimension to treatment planning. The variation of radiation quality, expressed by RBE values, carries the risk of systematic relative misestimations, for instance due to biological dose gradients in the tumour volume. This is especially relevant for large tumours in which the variation of radiation quality over the tumour volume is larger. On the other hand, high-LET radiation is generally less sensitive to changes in the radiosensitivity of biological systems compared to low-LET radiation, as explained earlier. Therefore, possible unwanted misestimations in cell survival and biological dose gradients in ion RT due to an overall under- or over-estimation of biological parameters which determine the cell radiosensitivity, such as αi and βi, should also be considered in relation to expected equivalent misestimations obtained for photons, i.e. αx and βx, if possible. In this paper, the impact of uncertainties related to some aspects of (i)–(iii) is addressed. Uncertainties in the modelling approach used for predicting cell survival and tissue response for ion irradiation (point (ii) and partly (i)) will be referred to in the following as uncertainty of type I, i.e. varying only the ion parameters αi and βi. In contrast, general uncertainties in the response of the biological system to both photon and ion irradiation (point (iii) and partly (i)) will be referred to as uncertainty of type II, e.g. varying αx or βx while accounting for the correlated variation of αi and βi. Aspects of uncertainties of fragment fluence spectra in carbon ion therapy and their impact on RBE (point (iv)) are discussed for instance by Lühr et al (2012).
3. Methods
In the context of the LEM (Elsässer et al 2010, and references therein), cell survival S(D) at a given dose D for photon and ion radiation is described by the linear–quadratic (LQ) model with an extension for high doses. LQ behaviour provides a reasonable description for photon doses up to about 5–30 Gy (depending on the biological system). A dose threshold parameter Dt defines the onset of a linear behaviour which is experimentally found at high photon doses (Astrahan 2008):
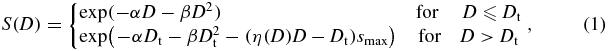
with the maximum slope smax = α + 2βDt and where η(D) ⩾ 1 quantifies damage clustering effects at very high doses (relevant above a few hundred gray), for details, see Elsässer et al (2007). Values of RBE and cell survival for ions for this study, are obtained by a re-implementation of the LEM (Elsässer et al 2010) developed at HIT. Unless stated explicitly otherwise, all references to the LEM in the following will refer to the HIT re-implementation of the LEM in the flavour of LEM-IV. A recent extension of the LEM model yields corrections to the LEM-IV for high doses, relevant for hypofractionation studies (Friedrich et al 2012). However, for doses up to about 2–3 Gy, predictions of the LEM-IV are judged to be sufficiently accurate for our purposes (Friedrich et al 2012).
The computation of three-dimensional biological dose and cell survival maps is performed with a MCTP research tool using the Monte Carlo code FLUKA (Battistoni et al 2007, Ferrari et al 2005) for the calculation of absorbed doses and fluences. RBE tables obtained from the re-implemented LEM are used to calculate biological doses using FLUKA (Mairani et al 2010). FLUKA is already successfully used for some time for re-calculations of ion RT treatment plans at state-of-the-art ion beam therapy facilities (Parodi et al 2009, Sommerer et al 2012). Using MCTP, optimization is performed with gradient-based inverse-treatment planning algorithms allowing single-field and simultaneous multiple-field optimization. Simultaneous optimization of multiple treatment fields is, in analogy to photon RT, often referred to as intensity-modulated particle therapy (IMPT) (Lomax 1999). Details about the MCTP research tool and the HIT re-implementation of the LEM are given elsewhere (Mairani et al 2012).
On the basis of the LEM, possible variations of the biological input parameters αx, βx and Dt, which characterize the radiosensitivity to photons, are propagated to obtain corresponding changes in predictions of the radiosensitivity for ions (αi and βi) for different tumours. This approach translates uncertainties for cell radiosensitivity in a consistent way for different radiation qualities. It evaluates equally the biological effect on cell populations in the treated volume with different radiosensitivities. Also the impact of variations of the LEM-internal biological parameters nuclear size11 rnuc and domain size ldom, which are determined from experimental data (Elsässer et al 2010), is evaluated for a survival level typical for a single treatment fraction. Such a sensitivity study reveals typical features of systematic false predictions occurring due to uncertainties in modelling parameters and allows one to relate the importance of the accuracy of individual parameters. It is important to point out that such a single-variate approach omits any form of correlation between parameters. It is generally assumed that the αx/βx-ratio is the most important characteristic of the photon response curve for the determination of clinical RBE values. Moreover, the αx/βx-ratio can be estimated from clinical data (e.g. Henderson et al 2009) and is often better known than the individual quantities αx and βx. As discussed by Scholz and Elsässer (2007), the RBE can be assumed to have a dependence on the intial-to-final photon slope ratio which involves the αx/βx-ratio as well as Dt. For these reasons, also correlated variations of αx and βx with a fixed αx/βx-ratio are evaluated in this study. Table 1 lists the nominal parameters for HSG, chordoma, liver and prostate cancer (Elsässer et al 2010, Henderson et al 2009, Tai et al 2008) as used in this work together with the introduced variations of the nominal parameters. The nominal parameters of different tumours indicate the spread of biological parameters found for tumours and their modelling. Chordoma as described in the framework of LEM-I (Krämer and Scholz 2000) is used for treatment planning with scanned carbon ions. It is compared in the following for different treatment situations with predictions of the LEM-IV version, which is generally found to describe experimental data better.
Table 1. Modelling parameters for the description of HSG, chordoma, prostate and liver cancer in the framework of the LEM together with the introduced single-parameter variations used in this study. Single-parameter variations are given as percentage of the nominal values. In addition to the variations shown in the table, αx and βx were also varied simultaneously with a fixed αx/βx-ratio.
| Parameters | αx (Gy−1) | βx (Gy−2) | αx/βx (Gy) | Dt (Gy) | rnuc (μm) | ldom (nm) |
|---|---|---|---|---|---|---|
| Cell line | Human salivary gland (HSG) | |||||
| Nominal | 0.313 | 0.0616 | 5 | 10.5 | 5 | 540 |
| Varied | ±5% | ±5% | ±5% | −33%/ + 50% | −20%/ + 25% | |
| ±25% | ±25% | ±25% | ||||
| ±50% | ±50% | ±50% | ||||
| Cell line | Chordoma (LEM-I) | |||||
| Nominal | 0.1 | 0.05 | 2 | 30 | 5 | – |
| Cell line | Chordoma | |||||
| Nominal | 0.0081 | 0.0033 | 2.45 | 30 | 5 | 540 |
| Varied | ±5% | ±5% | ±5% | −33%/ + 50% | ||
| ±25% | ±25% | ±25% | ||||
| ±50% | ±50% | ±50% | ||||
| Cell line | Prostate | |||||
| Nominal | 0.15 | 0.0682 | 2.2 | 30 | 5 | 540 |
| Varied | ±5% | ±5% | ±5% | |||
| ±25% | ±25% | ±25% | ||||
| ±50% | ±50% | ±50% | ||||
| Cell line | Liver | |||||
| Nominal | 0.01 | 0.000 67 | 15 | 30 | 5 | 540 |
| Varied | ±5% | ±5% | ±5% | |||
| ±25% | ±25% | ±25% | ||||
| ±50% | ±50% | ±50% | ||||
Due to the mixed-LET and particle spectra for clinical treatment fields, systematic changes in radiosensitivity may result in more or less severe impact in terms of conformity and misestimation of biological doses for these treatment fields. The influence of parameter variations on the obtained biological doses and survival levels is therefore evaluated for typical carbon ion treatment fields. Carbon ion treatments with single- and opposed-field geometry, with target sizes between 2 cm × 2 cm × 2 cm and 8 cm × 8 cm × 8 cm and depths in water between 5 and 25 cm are investigated. The impact of biological uncertainties on treatments is evaluated in the PTV by means of DVHs and using the quantities: mean deviation from the prescribed biological dose Ddevavg = Davg − Dtar, near maximum dose deviation Ddev1 = max (|D99 − Dtar|, |D1 − Dtar|) and inhomogeneity coefficient I1 = D1 − D99. The near maximum dose D1 and near minimum dose D99 are defined, respectively, as the biological dose received by 1% and 99% of the volume in the cumulative DVH. Dtar is the prescribed biological dose and Davg is the actual mean biological dose in the PTV.
For treatment plans using multiple fields, there are additional degrees of freedom which allows one to achieve a uniform dose in the PTV while optimizing other quantities, such as doses to OARs. Most patients treated with scanned carbon ions are irradiated with an opposed beam geometry (Jäkel et al 2001). In this work, treatment plans with opposed beam geometry were obtained in water with different optimization objectives. Optimizations were performed using three different strategies, each representing an extreme on the scales of trade-offs for opposed beams between (1) minimum integral dose to normal tissues, (2) robustness to physical uncertainties in terms of range uncertainties, patient positioning, patient motion and (3) biological robustness in the PTV. Minimum integral doses (MID) were obtained by optimizing explicitly for a low biological dose in the normal tissues surrounding the PTV. The distal-edge tracking technique is aimed in a similar way at the reduction of the integral dose (Lomax 1999, Oelfke and Bortfeld 2000). However, distal-edge tracking is only applicable with reasonable homogeneity in the PTV for a larger number of beam ports (>3). The sensitivity of treatment plans to physical uncertainties can be decreased by single fields with uniform biological doses (SFUD). Possible shifts of the individual treatment fields then result mostly in an offset of the homogeneous biological dose delivered by the field, but not in hot- and cold-spots in the clinical target volume (CTV). Homogeneous coverage of the CTV edges is assured by extended margins, included in the PTV. A straightforward method to decrease the sensitivity of treatment plans to biological modelling is to achieve a more homogeneous radiation quality in the target volume. By delivering for a given treatment protocol a more homogeneous radiation quality to the PTV which is independent of parameters of the treatment plan, such as size and depth of the SOBP, the risk for relative misestimations can be reduced. The same is true for OARs in vicinity. In the following, RBE was used as a measure for radiation quality and treatment plans were obtained while optimizing simultaneously for a constant biological dose and a constant RBE in the PTV (CRP).
4. Results and discussion
4.1. Impact of uncertainties in modelling parameters on RBE and cell survival
The consequences of uncertainties of LEM input parameters (type I) are presented for HSG cells irradiated with carbon ions. Figures 2 and 3 show the relative changes in RBE at 30% survival as a function of the remaining particle range in the continuous slowing down approximation (CSDA) in water for the varied parameters αx, βx, Dt, rnuc and ldom, see table 1. The varied RBE at a given remaining range (i.e. for a given carbon ion energy) was calculated for a carbon ion dose which results for the nominal parameters in a survival fraction of 30%. A survival level of 30% is approximately reached for HSG cells with a 3 GyE fraction.
Figure 2. Changes in varied RBE (type I) relative to the nominal RBE value at 30% survival as a function of the CSDA remaining particle range in water for HSG cells irradiated with carbon ions. The varied parameters are αx and βx.
Download figure:
Standard imageFigure 3. Changes in varied RBE (type I) relative to the nominal RBE value at 30% survival as a function of the CSDA remaining particle range in water for HSG cells irradiated with carbon ions. The varied parameters are Dt, rnuc and ldom.
Download figure:
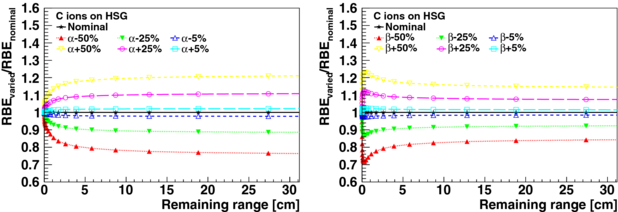
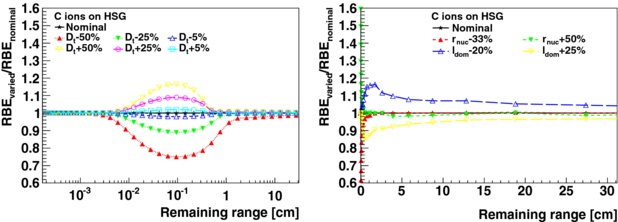
Standard imageVariations of αx and βx result in systematic changes of RBE values of carbon ions over their whole penetration depths. The RBE variation is nearly constant for remaining carbon ranges larger than 5 cm (lower LET region). In the last 5 cm of their remaining range (higher LET region), significant relative variations occur. As expected, the Dt threshold value is mostly relevant for the last millimetres of the remaining carbon ion range, where large local ion doses are reached. The consequences of the variation of the intrinsic LEM parameters rnuc and ldom as a function of the remaining range is depicted in figure 3. The areas of the cell nucleus of various human cell lines were measured by Suzuki et al (2000). For these measurements, the mean and maximum standard deviation of the nuclear radii rnuc within a cell line are found to be 8% and 15%, respectively. For the 16 measured cell lines, a standard deviation of 9% and a range from −15% to 16% of the mean of the nuclear radii are obtained. Variations of rnuc by −33% and +50%, which can be considered an estimated maximum range of uncertainty for this parameter, introduce only significant changes in RBE at the very end of the remaining range (<1 cm). In contrast, the variations of the domain size ldom by −20% and +25% lead to significant and non-constant misestimations of the RBE over the entire plotted remaining range. For lower survival fractions (i.e. 10% and 1%, not shown), as relevant for hypo-fractionated treatments, the behaviour of changes in RBE for parameter variations, as shown in figures 2 and 3 for 30% survival, remains essentially the same. However for Dt variations, changes of RBE are found to extend to larger remaining carbon ion ranges.
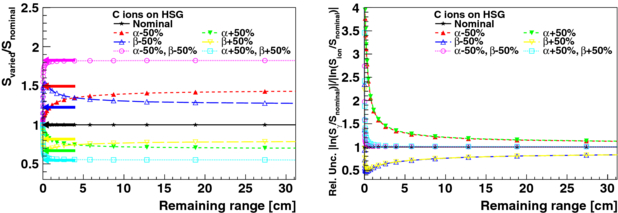
Uncertainties in the radiosensitivity of the treated biological system (type II) have an impact on both radiation response to photons and ions. Figure 4 (left) shows relative differences to the nominal survival Snominal = 30% for carbon ion irradiation Sion in comparison with photon irradiation Sγ for a variation of the nominal αx and βx by ±50%. According to the predictions by the LEM for HSG, it is found that the relative uncertainty (type II) in cell survival for a variation of αx is larger for photons compared to carbon ions, see figure 4 (right). Instead, for a variation of βx the opposite is the case. This is explained by the larger importance of βx compared to αx for ion irradiation for the description of the high local doses. Therefore, it is also found that the relative variation in survival increases further for a variation of βx towards the end of the remaining carbon ion ranges, where higher local doses are expected. As discussed earlier, the ratio αx/βx can be usually better determined from clinical data than the single parameters αx and βx and is decisive for the RBE. Therefore, it is of interest to also evaluate correlated variations of αx and βx (±50%) with a fixed αx/βx-ratio, see figure 4. For variations of αx and βx with the same sign, changes in survival add and result in a nearly constant shift of survival over the whole remaining particle range, except for the very end of the remaining range (<1 mm), where the parameter Dt is expected to become important. This variation of survival for ions is equal to the variation of survival for photons and results in a non-varying RBE. Thus, for a correlated variation of αx and βx with a fixed αx/βx-ratio, the relative uncertainty for photon irradiation is equal to the one for carbon ions apart from very short remaining ranges.
Figure 4. Left: changes in varied survival relative to the survival of Snominal = 30% for nominal HSG parameters as a function of the CSDA remaining particle range in water for HSG cells irradiated with carbon ions. Single and combined parameter variations of the nominal αx and βx by ±50% are shown. Arrows mark the change in cell survival for a corresponding variation of αx and βx for photon irradiation of HSG cells. Right: relative uncertainty (type II) in survival for photon irradiation compared to carbon ion irradiation is shown for single and combined parameter variations of the nominal HSG αx and βx by ±50%. Relative differences to the nominal survival Snominal = 30% are shown for survival for carbon ion irradiation with varied parameters (Sion) in comparison with survival for photon irradiation with varied parameters (Sγ).
Download figure:
Standard imageWith an increasing αx/βx-ratio (i.e. liver, not shown), the onset of relative misestimations of RBE for variations of αx or βx as a function of remaining range shifts to smaller remaining ranges. Instead, for smaller αx/βx-ratios (i.e. prostate, not shown), the onset of relative misestimations hardly changes. Dt is clinically not relevant for photons for standard fractionation schemes of 2 Gy. Instead, it is significant in the frame of the LEM for ions (and potentially for photon hypofractionation treatments (Astrahan 2008)). Hence, uncertainties in survival for a variation of Dt are evidently always larger for carbon ions compared to clinically relevant photon doses. As pointed out before, changes due to Dt are occurring predominantly only in the last millimetres of the remaining carbon ion ranges. For input parameters of liver, with a high αx/βx-ratio, a notable impact of variations in Dt is increased to larger remaining carbon ion ranges up to about 2 cm. Dt is known to be one of the critical input parameters for the LEM model, since reliable experimental photon survival data at the relevant high doses are scarce due to experimental difficulties in the high-dose region. Consequently, uncertainties for this parameter can be substantial.
Figure 5 illustrates the spread of survival for the same absorbed doses as a function of LET and CSDA remaining range for carbon ions and for photons for different tumours which are treated using carbon ions. Doses were chosen such that a survival Sreference of 30% was reached for chordoma. Since high doses are not well predicted by the employed LEM version (Friedrich et al 2012), values where high ion doses are need to achieve Sreference can be only regarded as indicative. However, it can be seen that, as already discussed in section 2, the spread of survival tends to be lower for high-LET radiation at the end of the remaining carbon ion range.
Figure 5. Spread of survival for the same absorbed doses as a function of LET and CSDA remaining ranges for carbon ions and for photons for different tumour types. Doses were chosen such that a survival Sreference of 30% was reached for chordoma. Arrows mark the relative survival for photon irradiation.
Download figure:
Standard image4.2. Impact of uncertainties in RBE on treatment plans
4.2.1. Sensitivity study for single fields
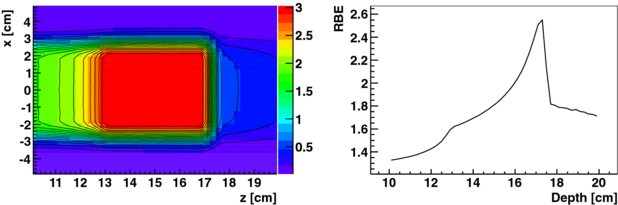
Carbon ion treatment fields in water optimized for nominal HSG parameters, see table 1, were computed for cubical PTVs with side lengths of 2, 4 and 8 cm at 5, 15 and 25 cm depths, respectively. Figure 6 shows the biological dose maps and the depth-RBE profile for nominal HSG parameters and a PTV of 64 cm3 at 15 cm depth.
Figure 6. Carbon ion treatment field for a cubical PTV of 4 × 4 × 4 cm3 at 15 cm depth in water optimized for nominal HSG parameters for a target dose in the PTV of 3 GyE. A biological dose map (left) and the RBE as a function of depth along the central axis of the field (right) is shown. The colour-scale is biological dose in GyE.
Download figure:
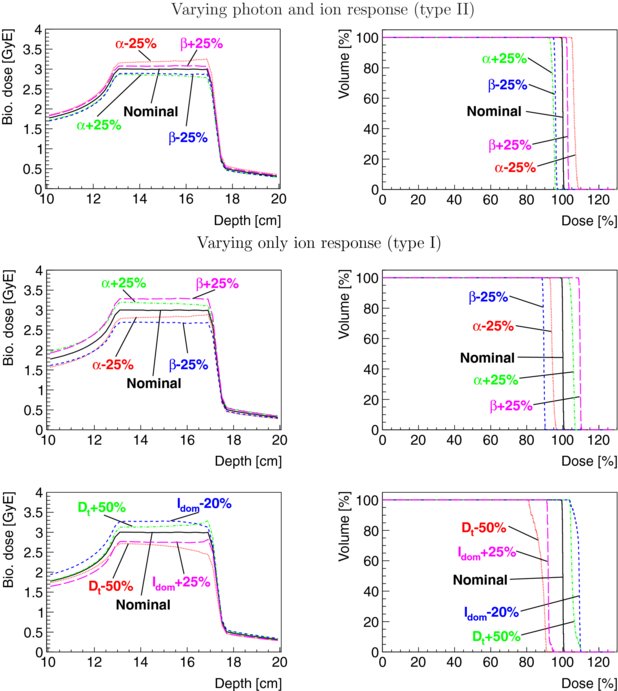
Standard imageChanges in biological doses due to the variation of nominal HSG parameters αx and βx by ±25%, Dt by ±50% and ldom by −20% and +25%, are shown in figure 7 in terms of depth–dose curves along the central axis of the field and DVHs of the PTV. In table 2, the impact of variations of biological parameters is evaluated for carbon ion treatment fields in terms of the mean biological dose deviation Ddevavg, near maximum dose deviation Ddev1 and inhomogeneity coefficient I1 in the PTV. I1 allows one to estimate the biological dose gradient introduced by parameter variations in the PTV. Biological dose gradients found for the treatment fields are generally smaller than the gradients observed in the relative RBE of carbon ions depicted in figures 2 and 3 for the same parameters. This can be mainly attributed to two factors: (1) fragmentation decreases the amount of high-LET carbon ions and gives rise to fluences of secondary particles with lower charges and LET (Böhlen et al 2010, 2012) and hence lower RBE variations and (2) a small given irradiated volume is traversed by ions of various energies and LETs which tend to average and thus reduce variation effects. Evaluating biological parameter variations in terms of biological dose (i.e. RBE differences) is adequate when the focus is set on possible misestimations in the modelling of biological response to ions with respect to the response of photons, which is assumed to be well known. This is done in the present evaluation by varying only the parameters determining the ion response (type I) and can reveal possible introduced gradients. However, this can be misleading when the focus is set on possible variations of the radiosensitivity of the irradiated biological system (type II) which affect both response to ion and photon irradiation. For this case, variations were introduced by varying both the parameters determining response to photons and ions. These types of variations manifest in a misestimation of the biological effect for both photon and ion irradiations and the relative effects are easier compared in terms of cell survival as previously shown in figure 4. Comparing the first and second row of figure 7 shows that the uncertainties in survival for the variation of αx are larger for photons than for ions. For uncertainties of type II, the misestimation of biological doses for βx is also considerably reduced.
Figure 7. Depth–dose curves and DVHs of a carbon ion treatment field for a cubical PTV of 4 × 4 × 4 cm3 at 15 cm depth in water optimized for nominal HSG parameters for a target dose in the PTV of 3 GyE are shown together with results for variations of the nominal HSG parameters αx and βx by ±25%, Dt by ±50% and ldom by −20% and +25%. For type II, both the αx and βx used for predicting the survival of ions via the LEM (resulting in αi and βi) and the αx and βx used for computing the RBE were varied. For type I, only the αx and βx used for predicting the survival of ions were varied while the nominal αx, βx for photons were used.
Download figure:
Standard imageFigure 8. Absorbed dose for a single beam port (left one), biological dose and RBE are shown as a function of depth for a cubical PTV of 4 × 4 × 4 cm3 at 15 cm depth in water and for a target dose in the PTV of 3 GyE. Treatment fields were optimized for chordoma parameters for a single field (SF) of carbon ions and for two opposed carbon ion fields using the different optimization objectives: minimum integral dose (MID), single-field uniform biological dose (SFUD) and constant RBE in the PTV (CRP). Distributions which were obtained for the particle fluences optimized for chordoma when using the biological parameters for other tumours (prostate, liver, HSG) are also displayed.
Download figure:
Standard imageTable 2. Sensitivity of single carbon ion treatment fields to variations of single HSG cell parameters (table 1). Treatment plans are optimized for nominal parameters and a prescribed dose of Dtar = 3 GyE. Resulting plans are compared to plans with varied parameters in terms of the mean dose deviation Ddevavg, near maximum dose deviation Ddev1 and inhomogeneity coefficient I1 in the PTV. Values are given as per cent of the prescribed biological dose Dtar. For the columns α⋆x and β⋆x, both photon and ion response were varied (type II). For all other parameter variations, only the ion response in the framework of the LEM was varied (type I).
| Varied parameters | |||||||
|---|---|---|---|---|---|---|---|
| Treatment scenario | Nominal | α⋆x (Gy−1) −25%/ +25% | β⋆x (Gy−2) −25%/ +25% | αx (Gy−1) −25%/ +25% | βx (Gy−2) −25%/ +25% | Dt (Gy) −50%/ +50% | ldom (nm) −20%/ +25% |
| PTV depth d = 15 cm, volume v = 64 cm3 | |||||||
| Ddevavg (%) | 0.0 | 6.4/-5.3 | −4.1/2.6 | −5.8/5.5 | −10.5/9.5 | −12.3/5.5 | 8.5/−8.0 |
| Ddev1 (%) | 0.7 | 8.6/7.2 | 5.0/3.4 | 7.1/6.8 | 11.4/10.4 | 18.8/10.2 | 9.9/8.8 |
| I1 (%) | 1.3 | 3.4/3.3 | 2.0/1.4 | 3.6/3.1 | 1.9/1.5 | 11.2/6.0 | 5.4/3.5 |
| PTV depth d = 5 cm, volume v = 64 cm3 | |||||||
| Ddevavg (%) | 0.0 | 6.8/-5.6 | −4.3/2.8 | −5.4/5.2 | −10.7/9.7 | −12.9/5.8 | –/– |
| Ddev1 (%) | 0.6 | 9.2/7.6 | 5.1/3.5 | 6.7/6.6 | 11.5/10.4 | 19.7/11.0 | –/– |
| I1 (%) | 1.1 | 3.6/3.5 | 1.9/1.4 | 3.8/3.3 | 1.8/1.3 | 11.9/6.5 | –/– |
| PTV depth d = 25 cm, volume v = 64 cm3 | |||||||
| Ddevavg (%) | 0.0 | 5.9/-5.3 | −4.1/2.3 | −6.2/5.5 | −10.6/9.2 | −12.2/5.2 | –/– |
| Ddev1 (%) | 0.8 | 8.3/7.2 | 5.5/3.3 | 7.4/6.8 | 11.8/10.2 | 19.1/9.8 | –/– |
| I1 (%) | 1.4 | 3.4/3.3 | 2.4/1.7 | 3.6/3.1 | 2.3/1.6 | 11.5/6.0 | –/– |
| PTV depth d = 15 cm, volume v = 8 cm3 | |||||||
| Ddevavg (%) | 0.0 | 7.2/-6.1 | −4.5/2.8 | −5.0/4.7 | −10.9/9.7 | −15.0/6.7 | –/– |
| Ddev1 (%) | 0.6 | 9.1/7.7 | 5.3/3.4 | 6.0/5.8 | 11.7/10.3 | 19.4/10.9 | –/– |
| I1 (%) | 1.2 | 2.7/2.8 | 1.8/1.6 | 2.8/2.6 | 1.8/1.5 | 9.3/5.5 | –/– |
| PTV depth d = 15 cm, volume v = 512 cm3 | |||||||
| Ddevavg (%) | 0.0 | 5.4/-4.4 | −3.5/2.4 | −6.7/6.5 | −10.1/9.3 | −10.0/4.5 | –/– |
| Ddev1 (%) | 1.5 | 9.5/7.6 | 5.2/4.1 | 8.5/8.3 | 11.6/11.2 | 18.9/10.0 | –/– |
| I1 (%) | 2.7 | 5.6/5.1 | 3.2/2.8 | 6.1/4.9 | 3.2/3.0 | 13.8/7.1 | –/– |
Entries marked as '–' were not evaluated.
For evaluated variations of the radiosensitivity (αx and βx variation, type II), a nearly constant shift in the PTV with only very slight biological dose gradients (larger for αx variation) is found for single carbon ion fields. For evaluated variations in the modelling of survival for ions (type I), also mostly constant shifts are found in terms of biological doses in the PTV for single carbon ion fields. The variations of αx, Dt and ldom introduce in addition biological dose gradients. Relative misestimations for varied parameters as a function of SOBP depth (5–25 cm) are small (generally <1% for the evaluated indices) (see table 2). It can be concluded that the increased nuclear fragmentation with depth has for the chosen scenario only a minor impact on misestimations of RBE for different SOBP depths. Instead, some larger relative misestimations (>1% for the evaluated indices) are found as a function of SOBP size. These relative misestimations are due to the increasing low-LET component in the PTV for larger SOBP sizes and PTV volumes.
4.2.2. Biological robustness of different multi-port optimization strategies
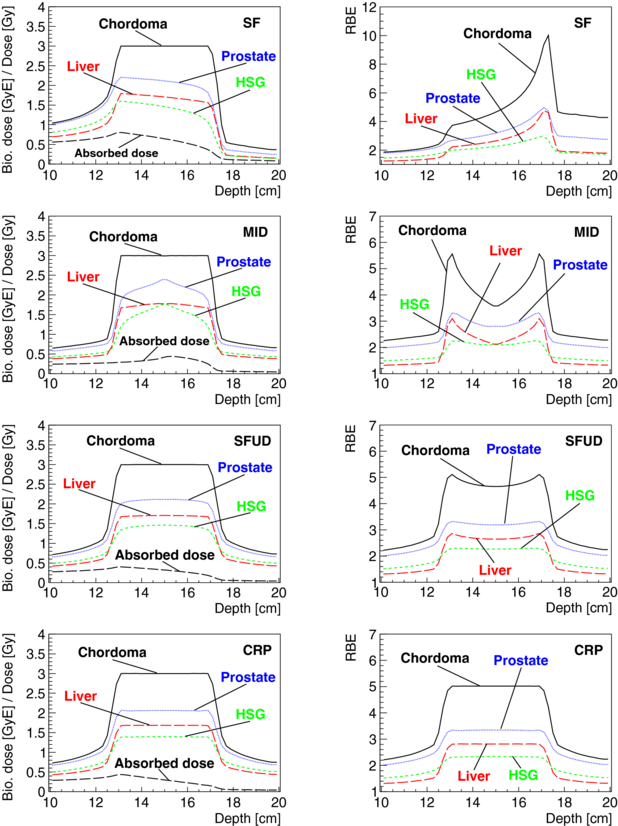
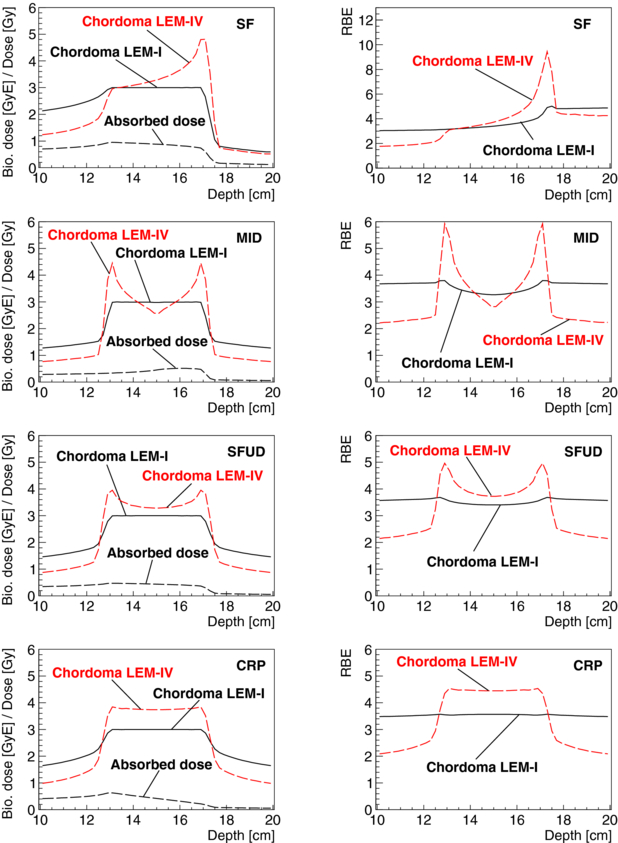
Advantages of opposed beam geometries in clinical settings are known and scanned carbon ion patients are mostly treated with opposed beams (Jäkel et al 2001). In contrast to carbon ion treatments delivered by single, orthogonal and patched fields, carbon ion treatments with an opposed beam geometry can be optimized to result in a constant mean RBE throughout the target volume. Treatment plans optimized for chordoma were calculated in water for a 64 cm3 cubical PTV using a single field and using opposed fields with the MID, SFUD and CRP optimization objectives (see section 3). Figure 8 shows the resulting biological doses and RBE distributions with depth for chordoma together with distributions which were obtained for particle fluences optimized for chordoma when using the biological parameters for other tumours (prostate, liver, HSG), see table 1. Figure 9 shows differences in biological dose and RBE predictions of the LEM for chordoma cells between model versions I and IV. Optimizations were done using LEM-I with corresponding chordoma parameters and are presented together with re-calculations for the same particle fluences using LEM-IV with corresponding chordoma parameters. For both figures, the absorbed dose for the left beam port is also displayed. In the case of irradiation with opposed fields, the absorbed dose delivered by the right field is symmetric.
Figure 9. Absorbed dose for a single beam port (left one), biological dose and RBE are shown as a function of depth for a cubical PTV of 4 × 4 × 4 cm3 at 15 cm depth in water and for a target dose in the PTV of 3 GyE. Treatment fields were optimized for chordoma parameters for LEM-I for a single field (SF) of carbon ions and for two opposed carbon ion fields using the different optimization techniques: minimum integral biological dose (MID), single-field uniform biological dose (SFUD) and constant RBE in the PTV (CRP). Distributions which were obtained for the particle fluences optimized for chordoma LEM-I when using the RBE values for chordoma LEM-IV are also displayed.
Download figure:
Standard imageAs expected and observable in both figures, biological dose gradients in the PTV for biological systems other than the system for which the treatment fields were optimized are significantly reduced for treatments with a more uniform radiation quality in the PTV. For opposed beams, biological dose gradients decrease in the PTV and homogeneity of radiation quality in the PTV increases for the different optimization criteria in the following order: MID, SFUD and CRP. For CRP optimizations, predictions of LEM-IV result in a nearly constant higher prediction of biological doses. For the shown SOBP size, this overprediction is about 25%. There are only minor biological dose gradients in the PTV. As expected, also the misestimation of the RBE in the entrance- and exit-channels is more constant for opposed fields compared to single-field irradiation. Range modifications at the distal edges of the SOBPs due to the varied RBE values are notable in both figures and are up to the scale of millimetres. For a more detailed discussion on range uncertainties due to variable RBE values for protons, see Carabe et al (2012).
Optimizing for a constant RBE in the PTV (CRP) for opposed beams results in two single fields with a ramp-like dose distribution which is decreasing in the PTV with depth. Very similar ramp-like dose distributions are used for carbon ion treatment planning (Krämer and Jäkel 2005). Compared to opposed fields with SFUD, it was found that CRP may allow one to significantly reduce doses to OARs for concave PTVs which enclose partially OARs (Krämer and Jäkel 2005). On the other hand, ramp-like dose distributions result generally in an increased entrance and integral dose compared to MID and SFUD. Treatment plans for ion therapy should be obtained trying to optimize the trade-offs between PTV coverage and homogeneity, biological doses to critical structures, the integral biological dose to the patient and considering aspects of treatment plan robustness. The robustness of treatment plans should be evaluated factoring in physical aspects, such as their sensitivity to range uncertainties, set-up uncertainties and sensitivity to motion, as well as the impact of biological uncertainties.
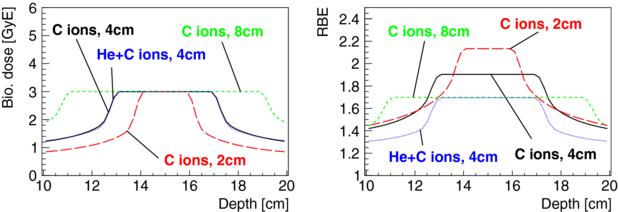
Treatment fields with a more uniform radiation quality in the PTV have reduced risks for biological dose gradients in the PTV, as shown using the CRP optimization. However, relative misestimations of biological dose can also occur as a function of the depth and size of the PTV (also PTV shape). These types of misestimations are more difficult to avoid. A possible way of reducing the risk for such hypothetical relative misestimations could be to treat the PTV for a given treatment protocol with a uniform radiation quality independent of size and depth of the PTV. Such an approach has similarities with proposals to irradiate biologically different tumour regions with tailored radiation qualities, often referred to as 'LET painting' (e.g. Bassler et al 2010). These approaches aim to treat a given tumour (region) with an optimal radiation quality rather than an optimal ion. When using a single ion species, RBE can be redistributed in the PTV, rather than being freely increased or decreased. Hence, the mean RBE in the PTV increases generally for smaller PTV sizes, see figure 10. A mixture of two ions can be used to achieve a more uniform radiation quality in the PTV also for different PTV sizes and depths. Figure 10 presents optimizations in which a constant RBE of 1.7 was obtained in the PTV for HSG cells by the mixture of helium and carbon ions for an opposed beam geometry for cubical PTV sizes of 64 and 512 cm3. The same RBE in the PTV as for a 512 cm3 PTV irradiated with carbon ions was obtained for a 64 cm3 PTV by giving 45% of the biological dose in the PTV with helium ions and 55% with carbon ions. In principle, the combined use of two ion species for a given beam port allows one to obtain a constant RBE in the PTV for treatment situations where the opposed field geometry is not advisable (e.g. treatments where orthogonal and patched fields are preferred). Evidently, it is debatable if it is of benefit to dilute the probably advantageous high-LET component of carbon ions in the PTV by the admixture of a lower LET beam. However, specifically for treatments with higher-LET ions, such as oxygen, the admixture of lower-Z ions could additionally help to reduce the fragmentation tail. Further, using a combination of different ion species is also an option for achieving a more uniform radiation quality throughout the PTV for treatments geometries which hinder the use of opposed fields. This includes treatments situations where orthogonal, patched or even single fields are preferable (Jäkel et al 2001). Nevertheless, even though the use of combined ion species is appealing in order to achieve a more constant and tailored radiation quality in the PTV, it remains technically challenging and increases treatment complexity, risks for motion and positioning uncertainties during the delivery of the different ion species as well as the overall treatment delivery time.
Figure 10. Biological dose and RBE as a function of depth for a cubical PTV of 8, 64 and 512 cm3 at 15 cm depth in water and for a target dose in the PTV of 3 GyE are shown. Opposed carbon ion treatment fields were optimized for HSG parameters to have a constant RBE in the PTV (CRP). Opposed fields consisting of helium and carbon ions were used to obtain the same RBE for a PTV of 64 cm3 as for a PTV of 512 cm3 which is irradiated only with carbon ions.
Download figure:
Standard image4.3. General remarks
Much of the presented analysis relies implicitly on the supposition that the translation of photon survival curves into ion survival curves can be in principal obtained in the mathematical framework of the LEM. This presumption is of course debatable and, as mentioned earlier, already in the LEM history from version I to IV the model was improved and extended to cope with systematic false predictions in describing experimental ion survival data based on photon survival curves. However, the typical behaviour of possible misestimations for the variation of input parameters, such as αx and βx, can be expected to be similar also for other biophysical models which are able to reproduce experimental data reasonably. Moreover, it is argued that the degree of possible relative misestimations of clinical RBE values inside the PTV and close by can be in general reduced by achieving a more uniform radiation quality in the PTV. For a constant radiation quality in the PTV for a given treatment protocol which is independent of other treatment parameters, such as notably the PTV size and shape, misestimations by the biological modelling may result merely in a constant false prediction of biological doses in the PTV (absolute misestimation). This eases the necessary accuracy needed for the biophysical modelling and its input parameters.
In this work, RBE is used as a substitute for physical quantities which determine the equivalence of a radiation field in terms of its biological effectiveness at a given point and dose. Instead of depending on biological quantities for assessing the homogeneity of radiation quality for treatment optimization, it is appealing to use physical quantities, such as LET or lineal energy, which are decoupled from biological modelling. Optimizations of carbon ion treatment plans for a constant LET in the target volume have been performed (e.g. Wilkens 2004, Bassler et al 2010). Lineal energy might be a more adequate choice for treatment plans involving several ion species, due to ambivalences of LET for different ions.
Some of the presented analysis was performed assuming that the treatment aim is to irradiate the PTV or a possible boost-region with a uniform biological dose as currently done for most patient treatments. There is an ongoing discussion in literature about the possible advantages of using tailored heterogeneous dose and LET distributions (e.g. Niemierko 1997, Wu et al 2002).
The possible systematic misestimations of RBE and cell survival, discussed in this work, should be extended to address more explicitly uncertainties in the prediction of biological effectiveness for tumours with a heterogeneous radiosensitivity. Prominently this is the case for (spatially distributed) hypoxia. The impact of hypoxia to biological uncertainties for treatment planning might be much larger than the effect expected from the discussed uncertainties and approximations such as the modelling of different tumours as the same tumour type. On the other hand, the distribution of hypoxic cells is generally non-constant and changing over the course of a treatment, thereby averaging systematic effects. Instead, the systematics discussed in this work are expected to change less. In this work, tumour radiosensitivity is always varied for the whole tumour and no inter-tumour variation of radiosensitivity is explicitly addressed as this would imply additional assumptions. However, the obtained resulting variations in biological dose, RBE or cell survival also allows one to estimate the impact on a hypothetical sub-population of the tumour with a different radiosensitivity.
5. Summary and conclusions
Systematic relative misestimations of RBE and survival are difficult to detect clinically. Such misestimations lead generally to a worse treatment outcome. Instead, absolute misestimations of RBE can be detected and corrected clinically more easily, for instance by dose escalation trials. The robustness of carbon ion treatment plans to uncertainties in biological modelling was investigated in form of a sensitivity analysis. For various tumours (HSG, chordoma, liver and prostate), variations of the input parameters αx, βx, Dt, nuclear size rnuc and domain size ldom were introduced (between 5 and 50%, depending on the model parameter, see table 1) and consequences on survival and RBE as a function of the remaining ion range were evaluated. For remaining carbon ion ranges associated with a lower LET (≳ 5 cm), uncertainties in biological parameters resulted predominantly in constant misestimations of survival and RBE (absolute misestimation). Furthermore, since low-LET ion radiation is more similar to photons, possible misestimations of RBE values for low LET can be assumed to be generally smaller. For remaining ranges in the higher LET region (≲ 5 cm), also varying misestimations of survival and RBE as a function of the remaining range were found. Consequently, the largest risk for relative misestimations is expected in the high-LET area inside and close to the PTV. For correlated variations of αx and βx with a fixed αx/βx-ratio, survival misestimations are constant over the whole remaining carbon ion range, except for the last millimetres where Dt becomes important. The radiosensitivity of cells to photons and ions was varied by varying αx and βx and obtaining the corresponding variation of αi and βi for ions according to the LEM. For variations of αx, this resulted in a larger variation of survival for photons compared to ions. For variations of βx, the opposite is the case.
For the investigated scenarios using single carbon ion fields, the conclusions were similar. For ion beam treatment fields, the RBE in a given voxel is determined by poly-energetic fluences of the primary ion beam and secondary charged fragments. It was shown that uncertainties in the biological modelling can introduce absolute and relative misestimations, for instance in the form of biological dose gradients in the PTV. However, it was found that moderate changes in the parameters of the biophysical model (see table 1) result mainly in misestimations of an absolute nature for single carbon ion treatment fields. Relative misestimations of the quantities: mean biological dose, inhomogeneity coefficient and near maximum dose deviation as a function of the SOBP depth were found to be for the tested cases mostly within 1% (for a constant SOBP size). Relative misestimation of RBE occurs as a function of the size of the SOBP. By increasing the homogeneity of the radiation quality in the PTV, the risk for relative misestimations in the form of biological dose gradients in the PTV can be significantly reduced. This eases the clinical importance of uncertainties in radiobiological treatment parameters and decreases the needed accuracy requirements for the biological modelling.
While two opposed carbon ion fields can result in rather stable conditions, single fields, patched fields and also two orthogonal beams introduce a larger risk for biological dose gradients in the tumour volume. By optimizing for a constant RBE in the PTV (CRP), a more homogeneous radiation quality is obtained in the PTV. Treatment plans optimized with such a criterion showed only constant shifts of RBE values in the PTV for variations of the input parameters of the biophysical model for HSG cells, for different representative tumours and for chordoma modelled by LEM-I and LEM-IV. Improvements of biological robustness for opposed beam geometries (using CRP optimization) occur at the expense of an increased entrance dose (compared to MID optimizations). Treatment fields with opposed beams which are more robust to physical uncertainties (using SFUD optimization) represent an intermediate solution in terms of integral dose and biological robustness. In general, the benefits of sparing normal tissues and critical structures should be balanced against physical and biological robustness for ion beam treatment plans. A detailed consideration of all these aspects is desirable for treatment planning. Treatment plans with a rather uniform radiation quality in the PTV (i.e. dose-ramps) are partly used clinically for opposed fields (Krämer and Jäkel 2005). Several strategies have been proposed to optimize IMPT treatment plans systematically with respect to uncertainties in physical parameters (Inaniwa et al 2011, Pflugfelder et al 2008, Unkelbach et al 2009). An extension to take systematically into account also biological uncertainties of ion beam treatment plans could help to improve the outcome of ion beam therapy.
In this study, comparisons were made for geometric cases with regularly-shaped target volumes and some examples of biological systems which allows one to investigate and outline principal aspects of biological uncertainties in ion beam therapy. Trade-offs in IMPT treatment plans between integral dose, doses to critical organs, physical and biological robustness (regarding the tumour and OARs) for different optimization criteria and techniques are probably best estimated based on some representative patient cases for a given treatment or on a case-to-case basis. For such an approach, a reliable estimation of uncertainties of the clinically used RBE values should be established.
Acknowledgments
The authors would like to thank Iuliana Toma-Daşu for helpful discussions and comments. This research project has been supported by a Marie Curie Initial Training Network Fellowship of the European Community's Seventh Framework Programme under contract number (PITN-GA-2008-215840-PARTNER).
Footnotes
- 10
This holds if assuming a tumour with a spatial uniform distribution of tumour stem cells and radiosensitivity, or assuming that these quantities are on average over many patients uniform but randomly distributed for individual patients and patient-specific distributions are not known, which is the rationale for delivering iso-dose or accordingly iso-biological dose treatments.
- 11
In the framework of the LEM, the nuclear size rnuc is an effective nuclear radius. It can be calculated as 80% of the average nuclear radius (Elsässer et al 2008b).