Abstract
Microelectronics play a crucial role in medical settings by monitoring physiological signals, treating illnesses, and enhancing human well-being. For implanted and wearable devices, a reliable and continuous energy source is essential. While conventional energy systems rely on batteries and external power connections, their drawbacks, including the need for frequent charging, limited battery lifespan, and the potential for reoperation, restrict their utility. This has spurred the exploration of self-sustaining, long-lasting power solutions. The ultrasound-driven nanogenerator, a promising energy source, harnesses biomechanical energy from activities like muscle movement, heartbeat, respiration, and gastric peristalsis. It converts this energy into electrical signals, enabling the detection of physiological and pathological markers, cardiac pacing, nerve stimulation, tissue repair, and weight management. In this review, we provide an overview of triboelectric (TENG) and piezoelectric (PENG) nanogenerator design with ultrasound and its applications in biomedicine, offering insights for the advancement of self-powered medical devices in the future. These devices hold potential for diverse applications, including wound treatment, nerve stimulation and regeneration, as well as charging batteries in implanted devices.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The ongoing progression and utilization of electronics alongside advanced materials have propelled the adoption of implantable and wearable electronic products, encompassing in vivo devices like pacemakers and neurostimulators, as well as smartwatches, eyeglasses, and bracelets [1–3]. Despite their considerable achievements, electronic devices encounter multiple challenges. In medical contexts, implanted devices necessitate small size, heightened flexibility, sensitivity, and mechanical stability to ensure optimal operation within the body [4–6]. The uninterrupted availability of power is crucial for the reliability of these electronic systems [7, 8].
Conventional power supply methods, which rely on batteries and external sources, suffer from drawbacks like bulkiness, high energy consumption, limited lifespan, and the need for frequent replacement [9–11]. These issues place additional financial, physical, and psychological burdens on patients. Addressing these limitations, the development of a self-powered electrical energy harvesting system with excellent biocompatibility and durability holds significance for medical electronic devices [12–14].
A nanogenerator is a device that converts micro, nanomechanical, and thermal energies into electrical energy, classified into three types: piezoelectric (PENGs) [15–17], triboelectric (TENGs) [18, 19], and pyroelectric (PyENGs) [20, 21]. PENGs and TENGs transform mechanical energy, while PyENGs convert thermal energy from temperature variations. Human body-generated thermal and mechanical energy is sufficient for biomedical nanogenerator applications. Originally proposed for self-powered nanodevices [22, 23], nanogenerators operate nanosystems independently, harvesting stray mechanical and thermal energy, and integrating easily with microelectronic devices. They are small, lightweight, simple to manufacture, and versatile, holding great potential for future intelligent and implantable electronic devices. Additionally, nanogenerators are cost-effective, use readily available materials, and are environmentally friendly [24]. Over the past decade, they have evolved from conceptual designs to practical technologies, harvesting energy from various sources like wind [25, 26], waves [27], and micromechanical movements such as muscle activity [28] and heartbeat [29].
In the realm of biomedical devices, nanogenerators assume a pivotal role in presenting a promising avenue for fulfilling the energy demands of diverse applications. Initially, as mentioned earlier, nanogenerators possess the capability to transduce mechanical energy, such as bodily movements or vibrations, into electrical energy. This functionality facilitates power generation from inherent bodily motions, obviating the necessity for external power sources or batteries, particularly advantageous for wearable devices. Secondly, nanogenerators can be seamlessly integrated into implants, including pacemakers or neural stimulators, to capture energy from internal bodily movements or mechanical processes. This diminishes the requirement for frequent battery replacements or reliance on external power sources, thereby augmenting the long-term sustainability of implantable medical devices (IMDs). Thirdly, nanogenerators commonly employ flexible and lightweight materials, rendering them suitable for integration into pliable and conformable biomedical devices. This flexibility enables seamless assimilation with the human body, fostering patient comfort and minimizing the likelihood of device rejection. Fourthly, self-powered nanogenerators can be deployed to furnish a continuous and sustainable power supply for biosensors and monitoring devices. Additionally, these nanogenerators can be designed to harness energy from biological processes, such as muscular motion or the circulation of bodily fluids. These devices find utility in real-time monitoring of physiological parameters, biomarkers, or drug levels without reliance on external power sources. Consequently, this technology harbors the potential to revolutionize the landscape of medical devices, rendering them more sustainable, reliable, and user-friendly in the foreseeable future.
In 2006, the world's first piezoelectric nanogenerator (PENG) based on a ZnO nanowire array was invented [33, 36]. This innovation generates an electric field through piezoelectric polarization, inducing electron movement and converting mechanical energy into electricity [37, 38]. Ultrasound-driven PENGs find utility in energy harvesting, sensors, wearables, and medical implants. Initially, materials such as ZnO, lead zirconate titanate (PZT), barium titanate (BT), and polyvinylidene fluoride (PVDF) were employed [39–42]. Subsequently, organic piezoelectric biomaterials, including protein nanofiber membranes, M13 phage membranes, and piezoelectric peptide nanostructures, were developed [43]. In 2012, a triboelectric nanogenerator (TENG) founded on triboelectricity and electrostatic induction principles was introduced [44, 45]. TENG leverages electrostatic charges produced at the interface of different materials, generating an electric field that propels electron movement. TENG outperforms in terms of output power and energy conversion efficiency, while offering flexibility in material selection. Capitalizing on everyday friction, ultrasound-driven TENG captures energy from natural surroundings like human motion, water droplets, airflow, ocean waves, and tides, enabling diverse applications [46–48]. Currently, TENG finds primary use in blue energy capture, human–computer interaction, wearable electronics, electronic skin, and IMDs [49, 50]. Within medical applications, TENG harnesses biomechanical energy from human muscle movement, heartbeat, and respiration for detecting physiological signals and treating ailments.
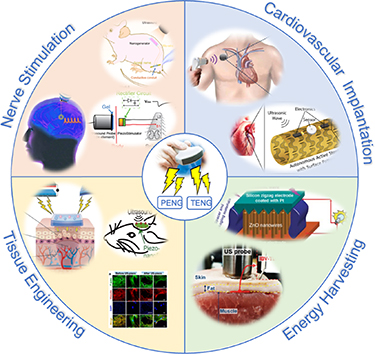
This review delves into ultrasound-driven nanogenerator design and operation principles, highlighting their applications in neuromodulation, cardiac monitoring, tissue repair, artificial retinas, motion correction, and training (figure 1). Furthermore, it outlines the future prospects of nanogenerators in the realm of biomedical engineering.
Figure 1. Biomedical applications of ultrasound-driven nanogenerators. From [30]. Reprinted with permission from AAAS. Reprinted from [31], Copyright (2022), with permission from Elsevier. © 2018 IEEE. Reprinted, with permission, from [32]. From [33]. Reprinted with permission from AAAS. Reprinted from [34], Copyright (2020), with permission from Elsevier. Reprinted from [24], Copyright (2023), with permission from Elsevier. Reprinted from [35], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution image2. TENG in biomedical field
Since Professor Z L Wang introduced it in 2012, the TENG has become a prominent player in the field of energy harvesting and sensor technologies [51–54]. TENG exhibits multifaceted capabilities—it can generate electrical signals in response to human body stimuli, functioning as an active sensor for real-time health information monitoring, and it can serve as a sustainable power source for medical devices [55–57]. The appeal of TENG is heightened by its cost-effectiveness, ease of manufacturing, versatile working modes, high energy conversion efficiency, extensive material options, and adaptable structure. These advantages position TENG as having significant potential in the realms of personalized healthcare and mobile therapy [18, 58–62]. Moreover, the electrical signals generated by TENG have direct applications in the stimulation of biological cells, nerves, tissues, and organs for electrical stimulation (ES) therapy [62–65]. Looking ahead, the integration of TENG with advanced technologies like cloud computing, big data, and artificial intelligence is poised to be instrumental. TENG and TENG-based body area networks are anticipated to play a crucial role in shaping the landscape of personalized medicine and mobile therapy.
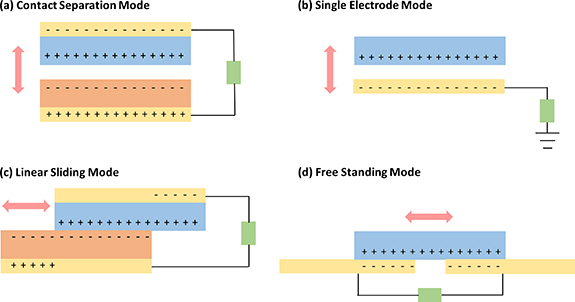
The TENG operates based on the principles of triboelectrification and electrostatic induction. Recent advancements in nanogenerators have led to the exploration of TENG's fundamental working modes, encompassing four main modes: vertical contact separation, linear sliding, single-electrode, and free-standing modes [66, 67] (figure 2). These modes necessitate two distinct triboelectric materials with appropriate electrode connections and insulation layers. Combinations can involve dielectric–dielectric or metal–dielectric setups. In all four modes, displacement within the triboelectric layers induces movement of electrostatic charges, disrupting the initial electrostatic equilibrium and resulting in a potential difference between the electrodes. Repeated mechanical action in forward and reverse directions causes the triboelectric layers to generate alternating potential between the electrodes, manifesting as positive and negative peaks in the TENG output, thus generating an AC signal.
Figure 2. Four operational modes of TENGs. (a) Contact separation mode. (b) Single electrode mode. (c) Linear sliding mode. (d) Free standing mode.
Download figure:
Standard image High-resolution image2.1. Contact separation mode
In the context of the contact separation mode TENG, triboelectrification is initiated through the interaction and subsequent parting of two triboelectric materials or layers (figure 2(a)). This phenomenon can occur between disparate dielectric materials or involve a combination of dielectric and metallic layers [68, 69]. This particular configuration offers notable benefits due to its uncomplicated design, straightforward manufacturing process, and economical nature. Additionally, this TENG mode holds the distinction of being the earliest developed and showcased method for energizing low-power electronics. It is worth noting that this TENG mode can also be employed in a stacked arrangement of multiple units to enhance overall output performance.
2.2. Single-electrode mode
The most basic configuration of TENG is the single-electrode mode, which, however, exhibits limited output performance due to its modest charge transfer capability [69]. Consequently, the resulting voltage and current generated are relatively low. Nevertheless, this mode proves highly appropriate for self-powered applications. An inherent benefit of this device lies in its ability to circumvent the limitations posed by the presence of contact wires obstructing both sides (figure 2(b)), a constraint encountered in contact separation mode and sliding mode TENG devices.
2.3. Linear sliding mode
In the linear sliding mode, charge generation occurs as a result of the relative back-and-forth motion between the layers of the TENG (figure 2(c)). The configuration closely resembles that of the contact separation mode, wherein electrodes are affixed to the rear of the triboelectric layers [69]. However, the distinctive characteristic here is the sideways displacement. When compared to the vertical contact separation mode, the sliding mode exhibits lower figure-of-merits due to the extended sideward motion during sliding. The merit of this mode lies in its capacity to yield heightened charge density, owing to the expansive contact area that enables efficient charge generation. Furthermore, the incorporation of additional grating structures can serve to amplify the output performance. It is important to note that the sliding mode TENG can also be adapted for rotational operation through the utilization of cylindrical grating structures.
2.4. Free standing mode
The free-standing configuration involves the unconfined movement of one electrode positioned amidst two electrodes or triboelectric layers (figure 2(d)) [69]. While the electrodes remain stationary, a triboelectric layer lacking an electrode is capable of gliding across them. This TENG mode exhibits elevated figure-of-merits and has exhibited notable efficiency in generating electrical output. Moreover, this particular device design lends itself to straightforward fabrication and seamless integration into diverse real-time applications.
3. PENG in biomedical field
The discovery of the PENG predates that of the TENG. The foundational operational concept of the earliest PENG was introduced in 2006, demonstrating the generation of electricity through the bending of a ZnO nanowire using an atomic force microscope probe [36, 70]. The essential principle behind PENG lies in the reciprocal exchange of mechanical and electrical energy within piezoelectric materials. When subjected to external force-induced deformation, polarization takes place within the material, leading to the simultaneous generation of positive and negative charges on opposing sides along the direction of polarization—a phenomenon known as the positive piezoelectric effect. Conversely, the inverse piezoelectric effect occurs when a voltage is applied to the polarization direction of a dielectric material, causing a phase change. PENGs effectively harness the positive piezoelectric effect in piezoelectric materials [71, 72].
Nanogenerators, categorized into three types—Piezoelectric (PENGs) [15–17], Triboelectric (TENGs) [18, 19], and Pyroelectric Nanogenerators (PyENGs) [20, 21]—operate based on distinct power generation mechanisms. While PENGs and TENGs convert mechanical energy into electrical energy [54, 73], PyENGs transform thermal energy resulting from temperature variations into electrical energy [74]. The notion of self-powered nanogenerators, rooted in the piezoelectric effect, was initially conceptualized in 2008 [23]. A nanogenerator serves as a device capable of harvesting incidental mechanical and thermal energies from its surroundings, converting them into electricity, thus functioning as a nanosystem independent of external power sources. This intrinsic capability provides an effective solution to address the power supply constraints of biomedical devices. Consequently, nanogenerators are regarded as an ideal energy source for various biomedical applications, encompassing power supply, sensing, treatment, microbial disinfection, and biodegradation.
Simultaneously, PENGs exhibit a unique set of characteristics that position them as highly promising in the field of biomedicine. Their biodegradability, compact size, lightweight construction, simplified manufacturing processes, and flexibility regarding fixed structures underscore their considerable potential for development. PENGs can seamlessly integrate with diverse microelectronic devices through various structural designs, enhancing their adaptability and versatility in biomedicine applications. Currently, PENGs find notable applications in two key areas within the medical field. They play a significant role in advancing self-powered health monitoring devices, offering a transformative solution to the energy challenges faced by healthcare technologies. By integrating PENGs with health-monitoring sensors, existing limitations related to powering healthcare devices can be effectively addressed [75–77]. Moreover, the exploration of PENGs for implantable applications has gained traction, capitalizing on the inherent biodegradable properties of piezoelectric materials [31, 78, 79]. This exploration holds promising prospects for the development of IMDs, offering a biocompatible and environmentally friendly alternative for sustained health interventions.
While the piezoelectric effect is conventionally associated with noncentrosymmetric crystals, analogous properties are evident in various materials, encompassing pyroelectric and ferroelectric substances. Under mechanical stress, piezoelectric materials undergo a modulation of their electric dipoles, leading to the separation of negative and positive charges across the material [80]. Similarly, pyroelectric materials demonstrate the capacity to induce polarization changes under external stimuli, with the transformation being proportionate to variations in temperature rather than mechanical stress. The adjustment in temperature causes subtle shifts in the positions of atoms within the crystal structure, consequently altering the material's polarization. Pyroelectric nanogenerators also find diverse applications in the biomedical field, including cryosurgery, skin cooling, and cancer treatment [81–84]. With the rapid evolution of wearable biomedical sensors and portable therapeutic devices, there is a burgeoning demand for pyroelectric materials characterized by high reliability, noise-free operation, and ease of handling.
4. Ultrasound-driven ES and electrical output
Ultrasound-driven ES refers to the use of ultrasound waves to stimulate tissues, leading to electrical changes within them. The ultrasound waves are acoustic waves with frequencies higher than the upper limit of human hearing. When ultrasound waves propagate through a medium, they create periodic compressions and rarefactions in that medium due to their vibrational nature. When these waves encounter biological tissues, they induce mechanical vibrations. These mechanical forces can cause ion movement or shifts within cells, leading to potential changes or even action potentials. The action potentials might propagate through neuronal or muscular tissues, leading to stimulation. This is similar to how electrodes might induce action potentials, but the difference here is that the stimulation is non-invasive and driven remotely by ultrasound, which can act as an initiator of transient processes.
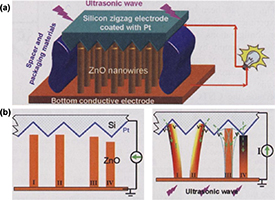
Apart from the above-mentioned mechanism, the vibrations generated by ultrasonic waves can also drive the physical movement of mechanical equipment components. Through appropriate device configuration, it made the designed device to trigger piezoelectric phenomenon or triboelectric reaction, thereby achieving the purpose of producing electrical output and cell stimulation. For instance, Wang et al developed the PENG which includes vertically aligned zinc oxide nanowire arrays, placed beneath a zigzag metal electrode with a small gap [33] (figure 3(a)). Under the excitation of ultrasonic wave, ZnO nanowires were asymmetrically compressed and relaxed by the electrode plate to generate a piezoelectric current via the external circuit (figure 3(b)). Additionally, Fan et al designed a TENG composed of two layers of thin films that contact and separate from each other because of acoustic vibration [85]. Triboelectric currents occur due to differences in film potential, it allows this TENG to not only transfer acoustic energy to electricity but also be used in acoustic wave sensors. Combining the ability of ultrasound to penetrate the surface of body into soft tissue with ultrasound-driven PENGs and TENGs, there are plenty of applications in human implantable devices to overcome the inconvenience of traditional electrical energy supply.
Figure 3. The mechanism of piezoelectric ZnO-nanowire nanogenerator driven by ultrasound. (a) Schematic diagram revealing the structure of the piezoelectric nanogenerator which includes vertically aligned zinc oxide nanowire arrays, placed beneath a zigzag metal electrode with a small gap. (b) Under the excitation of ultrasonic wave, ZnO nanowires were asymmetrically compressed and relaxed by the electrode plate to generate a piezoelectric current via the external circuit. From [33]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageMoreover, ES has found extensive application in facilitating the repair of various damaged tissues, including nerves, muscles, skin, and bones [53, 86, 87]. For instance, a wound care system combining ES with an aligned bacterial cellulose/gelatin membrane has demonstrated the ability to synergistically influence the migration of NIH3T3 cells. This approach significantly enhances the wound healing process by expediting wound closure, increasing granulation tissue thickness, promoting collagen deposition, angiogenesis, and activating key genes such as α-SMA, AKT, and ERK [88]. In the realm of nerve regeneration, studies have shown that the use of polypyrrole/silk fibroin conductive nerve conduits in conjunction with ES effectively fosters axonal regeneration and remyelination in vivo. In addition, ES applied to the conductive conduit activates the MAPKs signal transduction pathway, further aiding in nerve repair [89]. Additionally, ES has proven valuable in enhancing osteoblast differentiation, attachment, and proliferation during bone tissue regeneration. Consequently, ES holds significant promise for various tissue engineering applications. However, a notable drawback of current ES techniques is the inherent risk of infection associated with transcutaneous leads. Besides, these methods often necessitate a second operation to remove the tethered electrical-interface components. There is an urgent need for the development of innovative ES techniques that eliminate the reliance on commercial bulky ES power suppliers and transcutaneous leads.
5. Biomedical application of ultrasound-driven TENG
The advanced TENG technology represents a significant breakthrough in the realm of polymer-based bio-devices, particularly in their applications within the healthcare and medical diagnostic systems. An intriguing aspect of this technology is its compatibility with ultrasound, which can be employed to remotely activate TENG devices implanted or positioned near specific target sites. When ultrasound waves interact with the TENG, they induce mechanical strain within the device, leading to the generation of electrical pulses. These biomedical devices incorporate a variety of polymer materials, which have proven highly effective in regulating physiological functions and monitoring critical health parameters such as blood pressure, respiratory rate, and nerve stimulation [90]. Furthermore, researchers have delved into the possibilities of utilizing ultrasound-driven TENG as a self-sustaining power source for these bio-devices, with a particular focus on wearable and implantable applications (as illustrated in table 1). This exploration has illuminated a diverse range of collaborative engineering opportunities and has unveiled the promising future potential of enhancing existing biological sensing devices.
Table 1. Application of ultrasound-driven triboelectric nanogenerators (TENG).
| Years | Application | Features | References |
|---|---|---|---|
| 2019 | Transcutaneous ultrasound energy Delivery | The voltage and current generated ex vivo by ultrasound energy transfer reached 2.4 V and 156 μA under porcine tissue. | [47] |
| 2023 | Surgical wound healing | The bio-adhesive TENG driven by ultrasound to provide E-field up to 0.86 kV m−1 for accelerate wound healing. | [91] |
| 2022 | Power supply for implantable medical devices | Tn ultrasound-driven implantable wireless TENG energy harvesting system provides a stable direct current voltage of 1.8 V with >1 mW continuous DC output power and >10 mW instantaneous power. | [92] |
| 2022 | Acoustic energy transmission | The electrical output through FBI-TENG was up to 4.61 V and 27.86 μA when subjected to an ultrasound intensity of 0.5 W cm−2. | [93] |
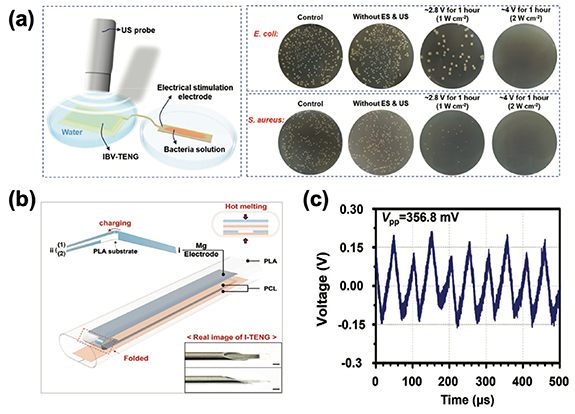
| 2023 | Inhibiting surgical site infection (SSI) | The biodegradable IBV-TENG eliminated nearly 100% of Staphylococcus aureus and 99% of Escherichia coli with the output of 4 V and 22 μA under ultrasound. | [94] |
| 2023 | Reduce the risks of implant-related injuries and infections | The injectable I-TENG is driven by ultrasound increasing cell migration and proliferation with minimally invasive wound. | [95] |
| 2023 | Materials that improve of energy transmission | 2-hydroxyethyl methacrylate (HEMA) be suitable for the surrounding environment, with ultrasonic transmission coefficient is 10 times higher than that of a titanium (Ti) plate. | [96] |
5.1. Surgical wound healing
Wound healing constitutes a multifaceted biological process encompassing distinct stages: Hemostasis, Inflammatory, Proliferative, and Remodeling [97, 98]. Simultaneously, the alternating electrical field stimulation facilitated by nanogenerator replicates the innate electric field's natural role in wound healing, promoting skin regeneration [99, 100]. Specifically, this intrinsic electric field emerges from the release of ions like Na+, Cl−, K+, and Ca2+ from injured cells or layers, generating an inherent potential at the wound site [101]. This regulates cell activity, particularly within keratinocyte-fibroblast cell clusters.
Electric stimulation emerges as a potent wound care strategy, mimicking the natural healing mechanism of endogenous electrical fields [91, 102]. Electric stimulation aids ion migration, growth factor production, neurite growth, cell migration, and proliferation [103]. Recent advancements in TENGs show promise for wound healing, utilizing simple structures to generate electricity through contact-separation movements of materials with different triboelectric properties. In the initial phase, the harvested energy flows as alternating current to two skin-attached electrodes positioned near the wound area [104]. Application of the AC electric field by these electrodes mimics the natural electric field, fostering regeneration of damaged tissue [105]. This encompasses cell migration, proliferation, and trans-differentiation, the key components of the four wound healing stages. Notably, TENG-driven electric field stimulation primarily influences the proliferative and remodeling stages. During proliferation, it promotes keratinocyte cell proliferation at the wound site, enhances fibroblast cell migration, and triggers myofibroblast trans-differentiation. Consequently, new granulation tissue forms in the dermal tissue, causing wound edges to contract. Similarly, in the remodeling stage, electric field stimulation aids fibroblast and myofibroblast cells in more rapid and precise wound site remodeling, facilitating re-epithelialization and scar tissue formation.
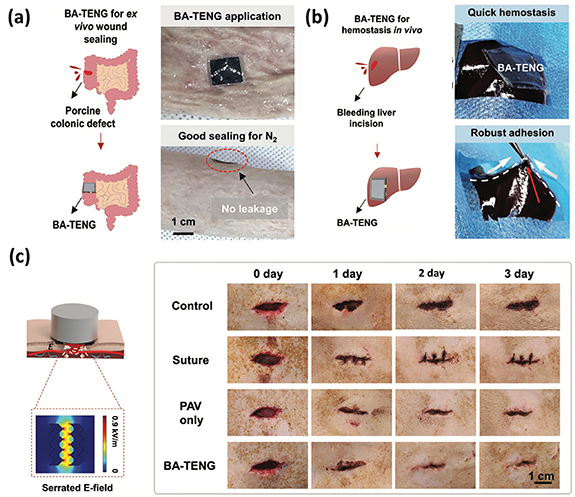
Ultrasound, more stable and effective than body movements, generates physical displacements for stimulation [106]. Acoustic waves penetrate the skin to reach deep body tissue, activating implantable and surface-attached TENGs to provide ES for wound healing. However, the presence of interfacial water on the surface of body tissue prevents effective interaction between tissue and TENG devices, making it challenging to secure the device on wet tissues and hindering ES. This limitation prevents the application of such devices for immediate treatment of acute wounds that require both robust sealing and instant hemostasis. Ultrasound driven bio-adhesive triboelectric nanogenerator (BA-TENG) was designed as a portable medical patch for rapid recovery of acute wounds on skin or organs, especially those with heavy bleeding and extensive trauma [100, 107–109]. The BA-TENG adhered robustly to wet tissue due to its bio-adhesive property and provides immediate wound sealing and hemostasis. Ultrasound application to the BA-TENG generates continuous electric fields, accelerating cell migration and proliferation for wound healing [110, 111]. As reported in the research conducted by Meng et al, they have developed a groundbreaking BA-TENG with the dual purpose of instant and robust wound sealing as well as accelerated wound healing through ultrasound stimulation [91].
Under ultrasound stimulation, the BA-TENG demonstrated impressive performance, producing a stable voltage of 1.50 V and a current of 24.20 μA even when submerged underwater. Experiments conducted on ex vivo porcine colon organ and in vivo bleeding liver incision have shown that the BA-TENG could seal wounds rapidly, within approximately 5 s (figure 4(a)) and was highly effective in achieving rapid wound closure and hemostasis, reducing blood loss by approximately 82% (figure 4(b)). In live rat applications, the BA-TENG not only provided immediate sealing of skin injuries but also generates a potent electric field (E-field) of about 0.86 kV m−1 when stimulated by ultrasound. This electric field significantly accelerated the healing of skin wounds (figure 4(c)).
Figure 4. The performance of BA-TENG in wound closure, hemostasis and promoting wound healing. (a) BA-TENG could seal wounds rapidly, within approximately 5 s on ex vivo porcine colon organ. (b) Highly effective in achieving rapid wound closure and hemostasis, reducing blood loss by approximately 82%. (c) In a live rat, the BA-TENG provided immediate sealing of skin injuries with a potent electric field (E-field) of about 0.86 kV m−1 when stimulated by ultrasound. [91] John Wiley & Sons. © 2023 Wiley-VCH GmbH.
Download figure:
Standard image High-resolution image5.2. Surgical site infection (SSI) prevention
In the quest to mitigate the heightened risks of SSIs, which significantly contribute to escalated rates of morbidity and mortality, the paramount objective is the eradication of microorganisms [52, 112]. Addressing the formidable global health challenge of antimicrobial resistance, the study by Imani et al presents an innovative approach for the eradication of deep-seated tissue microorganisms [94]. In their study, they introduced an approach to combat deep tissue infections using ES through an ultrasound (US)-driven implantable, biodegradable, and highly responsive triboelectric nanogenerator (IBV-TENG). The IBV-TENG generated an impressive voltage of approximately 4 V and a current of around 22 μA when subjected to ultrasound in vitro. The variation in electrical potential between the electrode surface and bacterial membrane disrupts electron transfer in bacterial activities due to electrostatic attraction. External electrical charge induces a negative charge on the bacteria membrane, altering its surface polarity. This polarization difference damages the bacteria structure, leading to leakage and, ultimately, bacterial death. These findings demonstrated its capability to deactivate nearly 100% of Staphylococcus aureus and approximately 99% of Escherichia coli, showcasing its effectiveness in combating microbial threats (figure 5(a)). Moreover, experiments conducted on porcine tissue samples ex vivo reveal that the IBV-TENG, when implanted, successfully neutralizes bacteria. This breakthrough antibacterial technology held significant promise as a strategic defense against SSIs, ultimately enhancing life expectancy and healthcare quality by safeguarding deep tissue against harmful microorganisms.
Figure 5. (a) In vitro evaluation of the antibacterial effect of IBV-TENG, demonstrating its capability to deactivate nearly 100% of S. aureus and approximately 99% of E. coli. (b) Schematic illustration of the structure of I-TENG. (c) Energy output of I-TENG driven by ultrasound (20 kHz, 1 W cm−2) in vivo. Reproduced from [94]. CC BY 4.0. © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. [95] John Wiley & Sons. © 2023 Wiley-VCH GmbH.
Download figure:
Standard image High-resolution imageTo mitigate the risks associated with implant-related injuries and infections, the study by Xiao et al have successfully developed an ultrasound-driven, biodegradable, and injectable triboelectric nanogenerator, referred to as I-TENG [95] (figure 5(b)). The beauty of this technology lies in its ease of administration through subcutaneous injection, minimizing the need for extensive incisions during implantation. During in vivo studies, the I-TENG exhibited remarkable stability, driven by ultrasound at a frequency of 20 kHz and a power density of 1 W cm−2. It produced a voltage of 356.8 mV and a current of 1.02 μA, demonstrating its reliable performance (figure 5(c)). Furthermore, in ex vivo experiments, the I-TENG generated an electric field measuring approximately 0.92 V mm−1. These findings are indicative of the device's capability to provide a consistent source of electrical energy. In addition, their cell scratch and proliferation assays have yielded promising results. They indicate that the electric field delivered by the I-TENG effectively promotes cell migration and proliferation. This suggests that the technology holds significant potential for expediting the healing process through the application of ES.
5.3. Ultrasound energy harvesting for medical devices
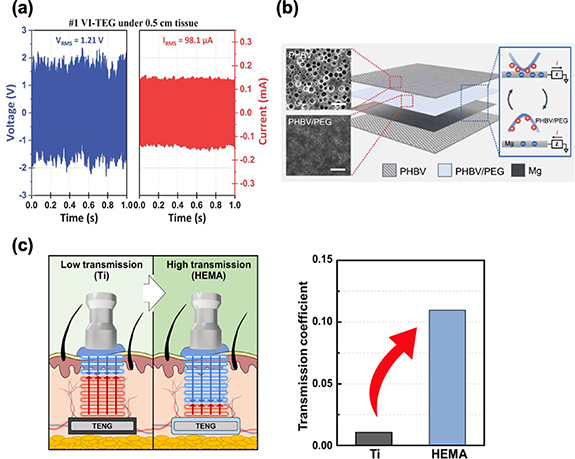
High-frequency acoustic energy carries significant energy potential and offers the ability to wirelessly transmit energy, presenting an exciting solution to the challenge of limited lifespan in IMDs [113, 114]. IMDs often necessitate regular surgical procedures for primary battery replacements, posing various risks and inconveniences. Then, the emergence of ultrasound-driven triboelectric energy harvesting technology provides a safe and efficient alternative for recharging IMD batteries. It is important to note that ultrasound technology has a proven track record of safety in medical applications, with over five decades of use in medical imaging and therapeutic procedures [47, 115]. Hinchet et al employed ultrasound as a method to transmit mechanical energy through both skin and liquids, and they presented a breakthrough in the form of a slim, vibrating and implantable triboelectric generator (VI-TEG) designed to efficiently capture this energy source [47]. The ultrasound waves have the remarkable ability to induce micrometer-scale displacement of a thin polymer membrane (perfluoroalkoxy, PFA), resulting in the generation of electrical energy through contact electrification. In practical tests, they demonstrated their technology's capability to recharge a lithium-ion battery at an impressive rate of 166 μC/s when immersed in water. Furthermore, when tested ex vivo using porcine tissue, they achieved notable voltage and current outputs of 2.4 V and 156 μA (figure 6(a)), respectively, through ultrasound energy transfer. Lee et al also introduced an innovative approach involving the utilization of biocompatible ultrasound sources to establish a remotely-triggered transient mechanism [93]. To demonstrate the viability of ultrasound-driven TENGs, they engineered a fully biodegradable and implantable TENG known as the FBI-TENG. This device comprised a magnesium (Mg) electrode layer, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) membranes, and a polyethylene glycol–based polymeric composite (PHBV/PEG) layer (figure 6(b)). Their primary objective was to assess the FBI-TENG's efficiency by modulating ultrasound intensity alone. Remarkably, the FBI-TENG exhibited an electrical output of 4.61 V and 27.86 μA when subjected to an ultrasound intensity of 0.5 W cm−2.
Figure 6. (a) The performance of VI-TEG ex vivo [47]. (b) Schematic demonstration of device structure (FBI-TENG) consisting of fully biodegradable materials: PHBV, PHBV/PEG, and Mg. (c) Ultrasonic transmission coefficient of HEMA approximately 10 times higher than that of a Ti plate. From [93]. Reprinted with permission from AAAS. Reprinted with permission from [96]. Copyright (2023) American Chemical Society.
Download figure:
Standard image High-resolution imageThese remarkable findings signify a groundbreaking achievement in the field, highlighting that their capacitive triboelectric electret technology stands as the first of its kind to rival piezoelectricity in harvesting ultrasound in vivo and powering medical implants.
Liu et al also developed a flexible ultrasound energy harvesting system designed for subcutaneous implantation [92]. This system fuses a TENG transducer with a power management circuit on a singular flexible printed circuit board. To optimize the performance of the TENG transducer, they selected an attached-electrode TENG with fine-tuned structural parameters. This adaptable system is versatile and can be used in a range of settings, and the improved version boasts an output power that's 66% stronger and has a reduced impedance compared to its predecessors. It consistently delivers a direct current voltage of 1.8 V with a continuous DC output power exceeding 1 mW and instantaneous power surpassing 10 mW. Such output levels are adequate to consistently power various sensors, run micromotors, and facilitate nerve stimulation.
Nonetheless, a significant portion of ultrasound energy is reflected when it encounters the surface of a titanium (Ti) encapsulation layer [116]. This layer is essential to safeguard against acute foreign body responses, inflammatory reactions, and necrosis triggered by unshielded materials [117]. Kim et al propose the use of multifunctional and biocompatible 2-hydroxyethyl methacrylate (HEMA) for both encapsulation and as a triboelectric layer in an implantable, modulus-tunable ultrasound-driven Triboelectric Nanogenerator (IMU-TENG) [96]. By adjusting the acoustic impedance of HEMA to match the surrounding environment, they achieved an ultrasonic transmission coefficient approximately 10 times higher than that of a titanium (Ti) plate (figure 6(c)). In in vivo conditions, the IMU-TENG generates sufficient energy to charge a 100 μF capacitor 3.7 times faster than when using a Ti plate. This innovative approach of utilizing multifunctional HEMA in high-performance ultrasound-driven TENGs holds significant promise as an energy solution for low-power IMDs, potentially extending their operational lifespan.
6. Biomedical application of ultrasound-driven PENG
Ultrasound technology has a longstanding presence in the field of medicine, serving dual roles in diagnostics and therapeutics [118]. Its ability to induce controlled thermal and mechanical effects within specific areas of the body has paved the way for tissue modification, repair, and, in some cases, targeted destruction. Concurrently, PENGs have found versatile applications across the biomedical spectrum, functioning as key components in sensors, actuators, and energy-harvesting systems [119]. A recent frontier in research involves the fusion of these established technologies. This novel approach centers on remotely activating PENGs using external ultrasound waves to generate electrical charges within bodily tissues. Here, ultrasound waves assume the role of mechanical catalysts, setting PENGs into motion and capitalizing on the direct piezoelectric effect. This burgeoning field holds immense potential for advancing non-invasive, precise, and highly effective biomedical interventions. Table 2 demonstrates the bio-medical application of ultrasound-driven PENG.
Table 2. Application of ultrasound-driven piezoelectric nanogenerators.
| Years | Application | Features | References |
|---|---|---|---|
| 2016 | To harvest ultrasonic energy powering a pacemaker within the human body | PUEH was constructed using a miniaturized PZT diaphragm array for extending the battery lifetime of a pacemaker. Distance fluctuation can also be overcome by adjusting new ultrasound frequency for the fluctuated distance. | [120] |
| 2018 | Powering platform for percutaneous coronary intervention | AAS, which was made by 50 μm-thick PVDF, harvested energy from ultrasonic waves and power up embedded sensors and electronics, monitoring and preventing potential failures. | [32] |
| 2019 | Retinal electrical stimulation | The device exhibited substantial current signals, surpassing the average thresholds required for retinal stimulation (e.g., current >72 μA and current density >9.2 nA μm−2). | [121] |
| 2020 | Activating the spinal cord neurocircuit | Ultrasound intensity as low as 0.1 mW cm−2 could induce MEPs in the hindlimbs. Piezoelectric stimulation induced by 22.5 mW cm−2 ultrasound restored locomotion in paralyzed rats with complete thoracic cord injury. | [122] |
| 2021 | Noninvasive manipulation of cell adhesion for cell harvesting | The PVDF/BTO film's surface potential became more negative, diminishing FN adsorption, influencing cell adhesive behavior, and changing the secondary structure of the adhesive protein FN from β-sheet to α-helix. | [35] |
| 2021 | Electrical stimulation of peripheral nerves | An ultrasound-responsive nanogenerator, based on piezoelectric composite thin films containing 0.5 Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3 (BZT-BCT) nanowires and PVDF polymer, served directly as neurostimulators. | [123] |
| 2022 | Electrical stimulation of peripheral nerves | An ultrasound-driven biodegradable PENG for the sustained delivery of in vivo ES to foster the healing of peripheral nerve injuries. The biodegradable PENG was composed of KNN nanowires, PLLA, and PHBV, as well as biodegradable encapsulation layers like PLA and PCL films. | [31] |
| 2022 | Deep brain stimulation | A piezoelectric ultrasound energy–harvesting device based on Sm-PMN-PT single crystal produced an instantaneous effective output power of 280 μW, which could immediately activate the periaqueductal gray brain area. | [30] |
| 2022 | A bone regeneration method, combining ES and tissue-engineering | Utilizing a biodegradable piezoelectric scaffold made of PLLA promoted osteogenic differentiation of stem cells in vitro and critical bone defect repair in vivo. | [34] |
PUEH, piezoelectric ultrasonic energy harvester; PZT, lead zirconate titanate; AAS, Autonomous Active Stent; PVDF, polyvinylidene fluoride; ES, electrical stimulation; KNN, potassium sodium niobate; PLLA, poly (L-lactic acid); PHBV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PLA, poly (lactic acid); PCL, poly- -caprolactone; Sm-PMN-PT, Sm-doped Pb(Mg1/3Nb2/3)O3-PbTiO3; BTO, BaTiO3; FN, fibronectin.
-caprolactone; Sm-PMN-PT, Sm-doped Pb(Mg1/3Nb2/3)O3-PbTiO3; BTO, BaTiO3; FN, fibronectin.
6.1. Ultrasound energy harvesting for medical devices
Implantable biomedical devices play a pivotal role in enhancing the quality of life for millions of patients across a spectrum of medical contexts, including applications like pacemakers, cardiac defibrillators, neural stimulators, and blood pressure monitors [124, 125]. However, traditional implantable biomedical devices currently rely on internal batteries for power, leading to the need for surgical battery replacement procedures. These procedures not only extend hospitalization periods but also expose patients to health risks, including elevated morbidity and even mortality. In response to these challenges, various energy harvesting technologies have been developed to extend battery life or potentially replace batteries altogether. Acoustic Energy Transfer (AET) stands out as a leading energy harvesting approach, facilitating the transmission of energy from an external source outside the body through propagating waves [126–128]. Importantly, AET offers the advantage of adaptable and stable output power, irrespective of factors such as the patient's size, the location of the implant, the shape of the organ, or organ vibrations. AET can effectively transfer energy even over considerable distances, even in the presence of a conductive medium, without causing any adverse effects on human tissue, rendering it highly suitable for implantable applications [129].
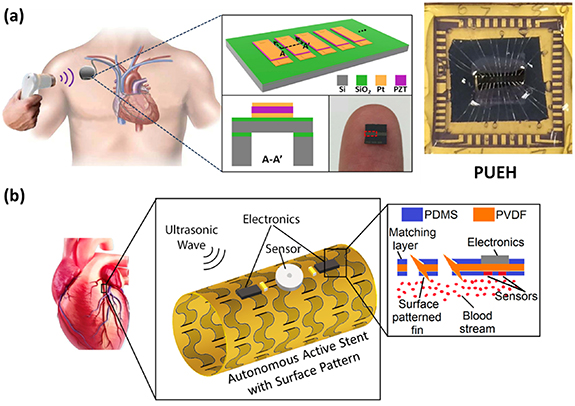
In a study by Shi et al, a novel Microelectromechanical systems (MEMS)-based broadband piezoelectric ultrasonic energy harvester (PUEH) was proposed with the aim of extending the battery life of pacemakers and other implantable biomedical devices [120]. Figure 7(a) illustrates the concept of the proposed PUEH powering a pacemaker within the human body. The PUEH is constructed using a miniaturized PZT diaphragm array, allowing seamless integration with an implantable pacemaker without significantly increasing its overall volume. The PUEH is designed to harvest ultrasonic energy from an external ultrasound source commonly employed in hospitals for diagnostic purposes, overcoming the limitations of Inductive Power Transfer (IPT) due to its inability to pass through the metal housing of a pacemaker. The optimized design of the thin PZT diaphragm results in the overlapping of two resonant models, providing an exceptionally wide operational bandwidth. Issues arising from standing waves and frequency loss can be mitigated by adjusting the ultrasound frequency for a given implanted distance. For instance, at a distance of 1 cm in water, the output power density can be increased from 0.59 μW cm2 to 3.75 μW cm−2 at an ultrasound intensity of 1 mW cm−2 by shifting the frequency from 250 kHz to 240 kHz. Additionally, distance fluctuations during the energy transfer process, caused by factors such as breathing or muscle movement, can be rectified through this frequency adjustment. For instance, when the distance deviates slightly (e.g., from 1 cm to 1.1 cm) at 370 kHz, the output power density experiences a significant drop from 4.10 μW cm−2 to 0.18 μW cm−2.
Figure 7. (a) Schematic of the PUEH integrated with a pacemaker and photograph of the PUEH on a human finger. (b) Schematic drawing of the Autonomous Active Stent, which is constructed using 50 μm-thick polyvinylidene fluoride (PVDF) with a range of surface patterns deliberately designed to facilitate expansion. Reproduced from [120]. CC BY 4.0. © 2018 IEEE. Reprinted, with permission, from [32].
Download figure:
Standard image High-resolution imageHowever, this power and efficiency loss can be restored to 4.06 μW cm−2 by adjusting the ultrasound frequency to 340 kHz. The authors professed the MEMS-based broadband PUEH exhibits tremendous potential as a power source for various implantable biomedical devices, offering versatility for a wide array of applications.
In another study conducted by Islam and Kim, an innovative ultrasonic energy harvesting system was introduced to serve as a power source for percutaneous coronary intervention (PCI) procedures, known as the Autonomous Active Stent (AAS) [32]. Figure 7(b) provides a schematic representation of the AAS. This cutting-edge device harnesses ultrasound energy to energize a variety of embedded sensors and electronics. It is poised to revolutionize the current PCI landscape, not only by replacing conventional bare wire stents but also by actively monitoring and preventing potential failures. The AAS is constructed using 50 μm-thick PVDF with a range of surface patterns deliberately designed to facilitate expansion. These surface patterns also enhance the AAS's ability to induce increased surface vibrations, thereby significantly improving overall power efficiency. Notably, AAS units with closely spaced surface patterns exhibited superior output, showing a remarkable 14.8% improvement compared to plain PVDF surfaces. With dimensions of 3 cm in length and a 1 cm diameter, the AAS demonstrated its capability to generate 230 μW of electrical power with an impressive efficiency rate of 11.5% when subjected to a 14 MHz ultrasonic wave.
6.2. Nervous tissue repairment
While it is currently no definitive cure for the paralysis and dysfunction resulting from nerve injuries, there is a ray of hope in the form of neuromuscular stimulation techniques such as functional electrical stimulation (FES) and epidural ES [130, 131]. These methods have shown promise in positively impacting functional restoration. Typically, neurostimulation involves the use of square pulse-shaped electric voltage or current generated by an electrical stimulator, which is either battery-powered or connected to an external power source [132]. However, research has indicated that certain non-rectangular waveforms can be more effective [133]. Regardless of the waveform shape used, these approaches generally require the implantation or connection of a neurostimulator to the patient's body.
Most implantable neurostimulators rely on batteries as their power source, which significantly increases the size of the implant [72]. Moreover, battery-powered stimulators necessitate a secondary surgical procedure when the battery's lifespan is projected to be exhausted, typically within 5–10 years, depending on usage patterns [134]. In contrast, some studies have explored the development of piezoelectric stimulators that do not rely on implanted power sources. Instead, these devices receive power through an ultrasound beam emitted from an external ultrasound probe [135]. This breakthrough has enabled the creation of a distributed stimulation system capable of delivering piezoelectric currents to the targeted organ.
Prior research has proposed that nerve ES exerts its effects through the up-regulation of certain key factors, including brain-derived neurotrophic factor (BDNF) [136], glial cell line-derived neurotrophic factor (GDNF) [137], Tyrosine Kinase receptor B (TrkB) [138], and the elevation of adenosine monophosphate (cAMP) [139]. ES initiates the influx of Ca2+ ions, leading to an increase in Ca2+ levels within nerve cells. This, in turn, triggers the up-regulation of BDNF and TrkB expression, consequently expediting axon regeneration via BDNF-mediated neurotrophin signaling [140]. According to the research of Chen et al, they delved into the direct ES of peripheral nerves using a soft piezoelectric thin film nanogenerator, which can be activated remotely through programmable ultrasound pulses [123]. They created an ultrasound-responsive nanogenerator, based on piezoelectric composite thin films containing 0.5 Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3(BZT-BCT) nanowires and PVDF polymer. Significantly, these piezoelectric thin film nanogenerators, operating without the need for additional rectifiers, can serve directly as neurostimulators. Furthermore, the electrical pulses generated by the implantable piezoelectric thin film could be precisely tailored through remote ultrasound excitation, allowing for adjustable input power and waveform control.
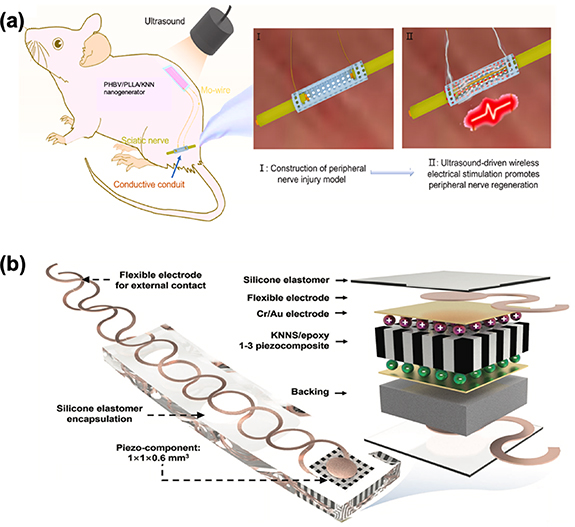
Taking a stride forward, Wu et al embarked on the exploration of biodegradable piezoelectric materials to enhance and monitor the repair of nerve tissue [31]. They demonstrated the application of an ultrasound-driven biodegradable PENG for the sustained delivery of in vivo ES to foster the healing of peripheral nerve injuries. As depicted in figure 8(a), when remotely stimulated by ultrasound with the appropriate power intensity, the implanted PENG could administer postoperative in vivo ES to augment nerve regeneration. The implantable PENG is composed of biodegradable piezoelectric materials, including potassium sodium niobate (KNN) nanowires, poly (L-lactic acid) (PLLA), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), as well as biodegradable encapsulation layers like Poly (lactic acid) (PLA) and poly- -caprolactone (PCL) films. By applying programmable ultrasound pulses to the subcutaneously implanted PHBV/PLLA/KNN PENG, adjustable ES parameters could be generated and applied to the biodegradable conductive nerve conduits, comprised of hydroxyethyl cellulose (HEC)/soy protein isolate (SPI)/black phosphorus (BP) nanosheets. The results unequivocally demonstrated that the effects on peripheral nerve repair in the ES group surpassed those in the non-ES group and were akin to the autograft group, which serves as the gold standard for peripheral nerve repair. Jiang et al introduced an innovative retinal ES component that harnesses ultrasound-driven wireless energy harvesting technology, transforming acoustic energy into electricity through the piezoelectric effect [121]. This breakthrough encompasses the design, fabrication, and performance of a millimeter-scale, flexible ultrasound patch employing a piezoelectric lead-free ceramic ([(K0.48Na0.52)(Nb0.95Sb0.05)-O3-(Bi0.4La0.1)(Na0.4Li0.1)ZrO3, abbreviated as KNNS), as illustrated in figure 8(b). To create the piezocomposite microstructure with enhanced electrical and acoustic properties, a modified dice-and-fill technique was employed. The resulting device can be securely attached to complex surfaces and activated by ultrasound to generate adjustable electrical outputs, achieving a peak output power of 45 mW cm−2. Moreover, in ex vivo experiments conducted in an implanted environment, the device exhibited substantial current signals, surpassing the average thresholds required for retinal stimulation (e.g. current >72 μA and current density >9.2 nA μm−2). These promising results indicate the device's potential integration into implanted biomedical devices for ES applications.
-caprolactone (PCL) films. By applying programmable ultrasound pulses to the subcutaneously implanted PHBV/PLLA/KNN PENG, adjustable ES parameters could be generated and applied to the biodegradable conductive nerve conduits, comprised of hydroxyethyl cellulose (HEC)/soy protein isolate (SPI)/black phosphorus (BP) nanosheets. The results unequivocally demonstrated that the effects on peripheral nerve repair in the ES group surpassed those in the non-ES group and were akin to the autograft group, which serves as the gold standard for peripheral nerve repair. Jiang et al introduced an innovative retinal ES component that harnesses ultrasound-driven wireless energy harvesting technology, transforming acoustic energy into electricity through the piezoelectric effect [121]. This breakthrough encompasses the design, fabrication, and performance of a millimeter-scale, flexible ultrasound patch employing a piezoelectric lead-free ceramic ([(K0.48Na0.52)(Nb0.95Sb0.05)-O3-(Bi0.4La0.1)(Na0.4Li0.1)ZrO3, abbreviated as KNNS), as illustrated in figure 8(b). To create the piezocomposite microstructure with enhanced electrical and acoustic properties, a modified dice-and-fill technique was employed. The resulting device can be securely attached to complex surfaces and activated by ultrasound to generate adjustable electrical outputs, achieving a peak output power of 45 mW cm−2. Moreover, in ex vivo experiments conducted in an implanted environment, the device exhibited substantial current signals, surpassing the average thresholds required for retinal stimulation (e.g. current >72 μA and current density >9.2 nA μm−2). These promising results indicate the device's potential integration into implanted biomedical devices for ES applications.
Figure 8. (a) Electrical stimulation administered by an ultrasound-driven biodegradable PHBV/PLLA/KNN piezoelectric nanogenerator enhanced the peripheral nerve repair. (b) Schematic illustration of the design, fabrication and key components of a millimeter-scale, flexible ultrasound patch employing a lead-free ceramic PENG. Reprinted from [31], Copyright (2022), with permission from Elsevier. [121] John Wiley & Sons. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
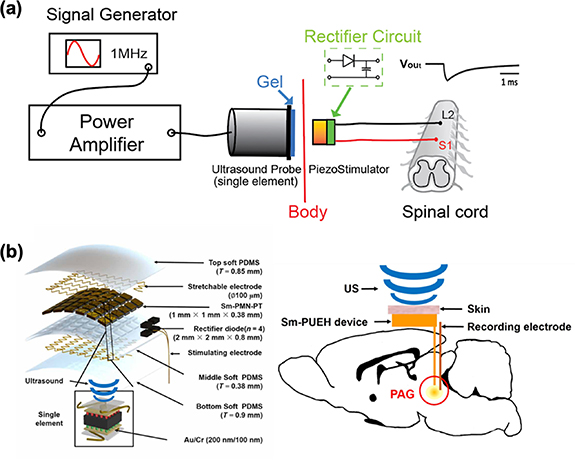
Standard image High-resolution imageElectrical stimulation (ES) for neuromodulation is a widely adopted technique used to address various neurological conditions related to the brain and spinal cord. In a study conducted by Li et al, the researchers investigated the effectiveness of piezoelectric stimulation (pES) using a customized miniature piezo stimulator to activate the neurocircuitry within the spinal cord [122]. They compared the outcomes of pES with those of conventional epidural ES in a rat model. As the illustration of figure 9(a) for their experiment, the researchers surgically implanted stimulation electrodes on the L2 and S1 spinal cord segments. These electrodes were connected to a head-plug for ES and linked to a piezo stimulator for pES. Additionally, EMG electrodes were implanted into hindlimb muscles to monitor muscle activity. To generate piezoelectric current, an external ultrasound probe delivered an ultrasound beam. The results indicated that ultrasound intensities as low as 0.1 mW cm−2 were sufficient to induce motor evoked potentials (MEPs) in the hindlimbs. Importantly, there were no significant differences observed in MEPs or muscle recruitment between ES and pES. Fascinatingly, akin to ES, pES induced by a 22.5 mW cm−2 ultrasound effectively restored locomotion in paralyzed rats with complete thoracic cord injuries. Further analysis of locomotion-related EMG signals indicated that pES exhibited a similar efficacy to ES. For deep brain stimulation, Zhang et al introduced a groundbreaking implantable piezoelectric ultrasound energy-harvesting device, utilizing Sm-doped Pb(Mg1/3Nb2/3)O3-PbTiO3 (Sm-PMN-PT) single crystal technology, drafted as figure 9(b) [30]. This innovative device exhibited an impressive output power density of up to 1.1 W cm−2 in vitro, marking a substantial advancement over the previous record of 60 mW cm−2, achieving a remarkable 18-fold increase. Upon implantation in the rat brain, this newly developed device demonstrated its capabilities under 1 MHz ultrasound with a safe intensity of 212 mW cm−2. It delivered an instantaneous effective output power of 280 μW, promptly activating the periaqueductal gray brain area. Rigorous rat electrophysiological experiments conducted under anesthesia, along with behavioral assessments, underscored the device's suitability for deep brain stimulation and analgesia applications.
Figure 9. (a) Schematic illustration of the piezoelectric stimulation (pES) through a subcutaneous piezo stimulator driven by acoustic energy with implanted stimulation electrodes on the L2 and S1 spinal cord segments. (b) Schematic diagram of in vivo experimental design of DBS using Sm-PUEH device implanted in rats and exploded view of Sm-PUEH device. Reproduced from [122]. CC BY 4.0. From [30]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution image6.3. Bone tissue engineering
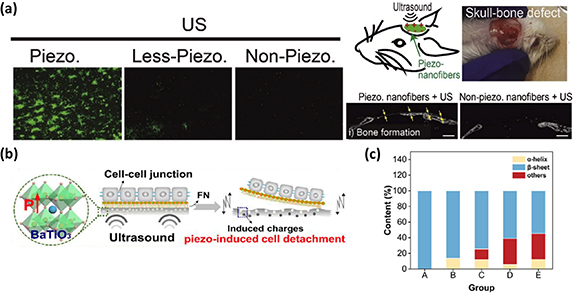
The reconstruction of significant bone defects poses a formidable challenge in modern medicine [141]. Historically, the gold standard for addressing such issues has involved the use of auto- or allografts. However, these approaches are suffered from limited tissue availability, donor site complications, infection, and the risk of immune rejection [142]. As an alternative, ES has garnered attention for bone regeneration [143]. Nevertheless, external electrical stimulators have proven to be less effective, while implanted devices rely on percutaneous wires and non-degradable toxic batteries. This reliance on non-degradable components necessitates invasive removal surgery. Addressing these challenges, Das and his colleagues introduced a bone regeneration method that combines ES and tissue-engineering approaches [34]. They achieved this by utilizing a biodegradable piezoelectric scaffold made of PLLA (Poly(L-lactic acid)), which, in conjunction with externally controlled ultrasound (US), generates surface charges to drive bone regeneration. Their research demonstrated that this approach, involving the piezoelectric scaffold and US, enhances the osteogenic differentiation of various stem cells in vitro and induces bone growth in a critical-sized calvaria defect in vivo, as illustrated in figure 10(a). This biodegradable piezoelectric scaffold, when combined with applied US, holds the potential to significantly impact the field of tissue engineering. It offers a novel, biodegradable, battery-free, and remotely controlled electrical stimulator, representing a promising advancement in bone regeneration techniques.
6.4. Cell modulation
Ultrasonic waves have become a prevalent mechanical force source in the realm of biomedicine, mainly due to their remarkable tissue-penetrating abilities and precise adjustability in terms of power, frequency, and duration [144]. In ultrasonic processes, the driving force acting on piezoelectric materials is primarily attributed to acoustic pressure and ultrasonic cavitation effects [145]. As the sound pressure gradually attains a critical value, minuscule gas bubble nuclei within liquids rapidly expand and abruptly collapse, generating shock waves. Ultrasonic cavitation involves dynamic processes, including expansion, collapse, and the formation of microjets. An intriguing application of ultrasound-mediated technology was demonstrated by Wan et al, who employed a piezoelectric composite film comprising PVDF and barium titanate (BaTiO3, BTO) for cell sheet harvesting (figure 10(b)) [35]. Under ultrasound stimulation (1 MHz, 0.8 W cm−2), the PVDF/BTO film exhibited impressive electrical properties, with an output voltage and current reaching approximately 100 mV and 0.19 nA, respectively. The adsorption and conformation of fibronectin (FN) are crucial factors in controlling early cell material adhesion. Following 20 s of ultrasound treatment, the PVDF/BTO film's surface potential became more negative, thus diminishing FN adsorption and influencing cell adhesive behavior. Simultaneously, the generated piezo potential under ultrasound stimulation could alter the secondary structure of the adhesive protein FN from β-sheet to α-helix. This alteration weakened interfacial protein interactions, further regulating cell adhesion behavior (figure 10(c)).
Figure 10. (a) A biodegradable piezoelectric scaffold made of PLLA induced osteogenesis in vitro and bone growth in a critical-sized calvaria defect in vivo. (b) Schematic diagram of a piezoelectric composite film comprising polyvinylidene fluoride (PVDF) and barium titanate (BaTiO3, BTO) for cell sheet harvesting. (c) The alteration of the FN secondary structure from β-sheet to α-helix, stimulated by the generated piezo potential under ultrasound. Reprinted from [34], Copyright (2020), with permission from Elsevier. Reprinted from [35], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution image7. Conclusion
In summary, the fusion of nanogenerators and ultrasound stimulation has emerged over the past decade as a revolutionary approach in various biomedical domains. These encompass neuromodulation, regenerative medicine, antibacterial therapy, and energy harvesting. However, despite the remarkable progress achieved thus far, there remain numerous opportunities and challenges that demand exploration and resolution in these critical medical fields. In addition to the considerations discussed earlier, future endeavors should include an exploration of targeting strategies, extensive biocompatibility studies, and the precise quantification of ultrasound-mediated piezoelectric effects. As we look ahead, it is clear that rapid advancements in cutting-edge technology and the continued development of piezoelectric and triboelectric materials will lead to the refinement and expansion of nanogenerators with these unique properties. This, in turn, will pave the way for their increasingly widespread implementation in clinical settings, fostering medical innovation and ultimately improving health outcomes.
Acknowledgments
This work was financially supported from the Young Scholar Fellowship Program by National Science and Technology Council of Taiwan (112-2636-E-002-012), the Medical Research Project of Chang Gung Memorial Hospital (CORPG3L0221)
Data availability statement
No new data were created or analyzed in this study.