Abstract
Magnesium implants that are biocompatible and biodegradable are important for orthopaedic applications. Mg-based alloys and their corrosion behavior have been studied in vitro and in a few in vivo studies. However, depending on the composition and microstructure, Mg-based alloys display varied biocompatibility, degradability, biocompatibility, and bioactivity. As a result, there is a critical need to create safe and cost-effective magnesium alloys for orthopaedic applications. The current investigation examined cytotoxicity, hemocompatibility, in vitro corrosion, and biomineralization of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites to establish its suitability as a biodegradable material. The biodegradation behaviour of pure Mg and its nanocomposites were investigated using a phosphate buffer solution. The Cytotoxicity of pure Mg and its nanocomposites were assessed using MG 63 cells in MTT (3-(4, 5-dimethyl-2-thiazolyl)−2,5 diphenyltetrazolium bromide) assays after 24 h. Biomineralization by MG 63 cells on pure Mg and its nanocomposites were analyzed using Alizarin red staining. The in vitro corrosion findings indicate more localized corrosion with rapid degradation on the surface of pure Mg and its nanocomposites. Pure Mg and its nanocomposites exhibited high hemolysis. The results of cytotoxicity showed high cell viability in pure Mg compared to its nanocomposites. According to the Alizarin red staining results, calcium was found to be deposited on the surface of Mg nanocomposites, and no calcium deposits on the pure Mg surface. The results of in vitro studies revealed that pure Mg and its nanocomposites responded differently in different tests. From these results, comparing Mg nanocomposites could be a more effective strategy to address the current challenges in orthopaedic implant applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Researchers are interested in employing magnesium (Mg) as a temporary implant for orthopaedic applications because of its distinctive properties [1–3]. Magnesium (Mg) is a low-density material with a high strength-to-weight ratio and has Young's modulus close to the natural bone (Mg: 1.78 g cm−3, 40–45 GPa; Cortical bone: 1.8 g cm−3, 10–27 GPa) [4–6]. Magnesium is bioresorbable and naturally degrades in the human body, unlike other metals like chromium, cobalt, nickel, and titanium [7, 8]. Because of these characteristics, it is ideal for load-bearing applications and fixation devices, including bone plates, screws, rods, and wires. Mg is the fourth-richest element in the human body after calcium, potassium, and sodium, which help in metabolic and enzymatic processes. It is predominantly found in the bone (60%–70%), and the remaining amounts are found in cells and blood vessels [9]. The clinical implementation of this implant is hampered by low ductility and degrades rapidly before the tissue heals [10–12]. Magnesium undergoes a complex corrosion process at the implant site that releases Mg (OH)2 and hydrogen gas [13, 14]. The formation of hydrogen gas bubbles delays the healing process at the fracture site, which ultimately results in necrosis because they prevent oxygen from reaching the surrounding tissues [15–17]. The current issue of using Magnesium implants is hydrogen gas evolution. The previous reports stated that hydrogen gas evolution in magnesium implants could be controlled by immersion test or anodic polarization method. Because the amorphous alloy surface with a more positive potential could retard the cathodic hydrogen evolution and the Mg dissolution that generates anodic hydrogen evolution. Decades back, for orthopaedic applications, temporary implants made of titanium, stainless steel, and cobalt-chromium alloys have been used for joint replacement and fracture treatment [18]. The drawback of the temporary implant is that it is nonbiodegradable and must be removed from the human body through a second procedure after the fracture tissue has healed [19]. Temporary implants that have been in contact with bodily fluids for an extended period of time may experience leaching, corrosion, or wear. This could eventually lead to implant failure [20–22]. Past and current research is mainly focused on creating biodegradable magnesium-based implants for orthopaedic applications [23–25].
According to previous research, pure Mg corrodes rapidly in the simulated body fluid due to a high chloride environment with pH 7.4–7.6 [26]. Alloying or surface treatment is one of the best ways to control the degradation rate. Rare Earth elements, manganese, zinc, and aluminium, are increasingly used as alloying elements. Alloying improves biocorrosion resistance while maintaining the mechanical and biocompatible properties of the material [27, 28]. The elements used for alloying should be biodegradable and non-toxic under physiologic conditions because cells cannot metabolize them. Previous studies reported that implant materials made of pure Mg, binary Mg series (such as Mg-Sr, Mg-Ag, Mg-La/Nd/Ce, Mg-Ca, and Mg-Zn), ternary and quaternary series (such as Mg-Al-Zn, Mg-Ca-Zn, Mg-Zn-Zr, Mg-Nd-Zn-Zr, etc) were tested for their effectiveness in fracture healing and other orthopaedic traumas [29–33]. Several studies have demonstrated the potential of magnesium alloys for orthopaedic applications, but corrosion is the major concern for Mg-based implants [34]. Using various techniques, it has become easier over time to analyze why magnesium-based implants fail. Even though alloying additions have resulted in improved corrosion resistance, the controlled degradation rate desired to use it for orthopaedic applications (3–6 months) is not achieved [18, 35–37].
Nanocomposites are the materials of the twenty-first century because they have unique features and property combinations that are not seen in conventional materials. In the last 20 years, a wider range of processing techniques has been used to develop nanomaterials, which are widely used in all applications [38]. Since the use of nanoparticles in medicine has expanded, many researchers have recently focused on developing new implants made of nanocomposite materials [39].
Ni/Ti is a suitable alternative to consider for materials in biomedical applications due to its characteristics, including excellent fatigue strength, high corrosion resistance, high damping capacities, and moderate to high impact resistance [40, 41]. Because of its improved functional performance, biocompatibility, and reduced elastic modulus that matches bone, NiTi is a popular material for surgical tools, orthodontic wires, and cardiovascular stents [42–45]. Nickel ions have been related to a potential risk in the human body, and it reported that biocompatibility was minimal or nonexistent. Adding a proper proportion of Ni/Ti to pure magnesium improves corrosion resistance and maintains good mechanical properties [40–43]. The Disintegrated Melt Deposition (DMD) technique was first developed by Gupta et al [46, 47], which is a modification of the spray atomization and deposition technique. NiTi is a reinforcement used for fabricating Mg metal matrix nanocomposites to improve mechanical and corrosion properties [48].
Mg 0.4Ce/ZnO2 nanocomposites were also fabricated by disintegrated melt deposition technique [49]. Previous studies have reported the addition of cerium in small amounts could improve the mechanical properties and corrosion resistance [50]. Previous research related to biocompatibility on Cerium (Ce) shows positive results on the proliferation and development of fibroblastic and osteoblastic cells. Ce can substitute calcium ions in biological molecules without compromising their function in the human body. Zinc nanoparticles have been widely used in engineering and science applications [51, 52]. The antimicrobial characteristics of ZnO NPs (antibacterial and antifungal) have piqued interest, particularly in the biomedical field [53].
Magnesium-based materials have been the focus of numerous in vitro and in vivo experiments [54–56]. For any material to function as an implant, it has to perform its biological role properly, as cell line studies are very important. Cell attachment and differentiation are significantly influenced by surface properties such as roughness and chemical composition of the material. Previous studies reported that In vitro cytocompatibility of Mg-based materials using osteoblast, L929 cells, and fibroblast cells increases cell death when treated with various doses of pure Mg [57]. Another study reported that the nickel ions exhibited cytotoxicity for different cell lines, such as osteoblastic cells, L929 cells, MG63 cells, and fibroblastic cells. These cell lines have shown no Cytotoxicity when the safe concentrations of nickel are less than 1.25 mg l−1 [58, 59]. The regulations for medical devices state that the inclusion of biocompatible material is safe up to 5 mg/l. Pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites are the materials used in the current investigation. In vitro and corrosion studies have been done to assess the biocompatibility of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites.
2. Materials and methods
2.1. Preparation of specimens
Partially or fully characterized nanocomposite Mg 0.4Ce/ZnO2 and Mg Ni/Ti were received from Dr M. Gupta, Associate Professor, Department of Mechanical Engineering, National University of Singapore. Pure magnesium with 99% was used for comparison with its nanocomposites. Pure magnesium with 99% purity has been used in this study. The composition of Mg 0.4Ce/ZnO2 nanocomposites with the major composition of Mg, 0.4% of Ce, and 1.5% ZnO2. The composition of Mg Ni/Ti nanocomposites with the major composition of Mg and 1% Ni/Ti nanoparticles. Using an electrical discharge machine, cast blocks were sliced into specimens that are cylindrical and have a diameter of 10 mm and a height of 4 mm. [Ratnaparkhi SC100]. Before being used in the experiments, all samples were sterilized.
2.2. Microstructure characterization
An Olympus metallurgical microscope (Olympus, Japan, BX51M-N33MD) was used for the microstructural investigation of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites. The samples were mounted, manually treated, and polished up to 1500 grit with Silicon carbide paper. Then the specimens were etched for 5–10 s in a picral solution containing 10 ml acetic acid, 4.2 g picric acid, 10 ml deionized water, and 70 ml ethanol [60].
2.3. Hemocompatibility assay
Fresh blood was taken from sheep and anticoagulated with 3.8 percent sodium citrate (the blood to sodium citrate ratio was 9:1), followed by centrifugation at 4 °C for 5 min at 4000 rpm [61]. The plasma and buffy coat layer were removed by aspiration. The red blood cells were centrifuged and washed three times in ten volumes of 10 mM phosphate buffer saline (PBS) adjusted to pH 7.4 by centrifugation (4000 rpm, 5 min). During each wash, the supernatant was eliminated. The erythrocytes were obtained by centrifugation (4000 rpm, 5 min) during the final wash. The erythrocytes were resuspended in PBS buffer and maintained at 4 °C until they reached the desired hematocrit level. An erythrocyte suspension (10% hematocrit) was treated with pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites in PBS for 2 h. After incubation, 200 ml of the reaction solutions were removed and diluted with 3.8 ml of PBS before centrifugation at 4000 rpm for 10 min. The absorbance of the resulting samples at 540 nm was determined with a spectrophotometer. The reaction mixtures were treated with the same amount of 3.8 ml distilled water to achieve complete hemolysis. The rate of hemolysis is measured using the following formula:
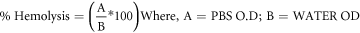
2.4. In-vitro degradation study
The degradation test was performed in phosphate buffer solution (PBS) at 37 °C in a static condition from the 3rd day to the 14th day [62]. PBS solution comprising 8g sodium chloride (NaCl), 0.2 g potassium chloride (KCl), 1.5 g disodium phosphate (Na2HPO4), and 0.2 g potassium dihydrogen phosphate (KH2PO4) pH was adjusted to 7.4 to imitate the physiological environment accurately. The deposited white layer as a result of degradation on the surface of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites was removed. Also, the soluble gets deposited at the bottom of the solution. The mass loss is determined by eliminating the products of corrosion from the surface of the samples using chromic acid (180 g l−1 in distilled water). Then the samples were cleaned using distilled water and air-dried. The surface morphology was determined using SEM on the dried samples.
2.5. Cytotoxicity assessment (MTT assay)
To assess the cytotoxicity of magnesium nanocomposites, MG63 cell lines obtained from National Centre for Cell Sciences (NCCS), Pune, were used. And determine the cell viability using MTT (3-(4, 5-dimethyl-2-thiazolyl)−2,5 diphenyltetrazolium bromide) assay [63]. The cells were cultured in DMEM with 10% FBS, 100 g ml−1 penicillin, and 100 g/ml streptomycin at 37 °C in a humidified condition of 50 g ml−1 CO2. The pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites samples were seeded in a 24-well plate with 1×105 cells per well and cultured for 24 h at 37 °C with 5% CO2. There were also controls with the MG63 cells and no magnesium discs. Then, 100 μl ml−1 per well of MTT with a concentration of 5 mg ml−1 in PBS was transferred into each well and incubated for 4 h. During incubation, the mitochondrial enzyme succinate dehydrogenase of viable cells cleaved MTT into water-insoluble, purple-colored formazan crystals. After carefully removing the MTT solution, the formazan crystals that had attached to the cells were released after being mixed with 1 ml of DMSO and incubated at 37 °C until complete solubilization of the crystals. The absorbance of samples was measured at 540 nm (A540) with UV Spectrophotometer and DMSO as a blank solution. All experiments were performed in triplicates, and the results were statistically analyzed using the Student's t-test for independent samples with a predetermined p = 0.01. The viability of cells was expressed as follows:
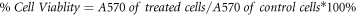
2.6. Biomineralization by alizarin red staining (ARS)
MG63 cells calcium accumulation was identified using ARS. The calcium generated by MG63 cells is bound by the ARS dye [64]. MG63 cells were seeded on pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites samples in a 6-well plate and incubated for 24 h. Before usage, the monolayer cells were cleaned in sterile PBS. The cells were fixed with 2% glutaraldehyde and then treated with ethanol. The cells were stained with 0.1 ml of 1 percent Alizarin red dye for one minute. After being treated with ARS dye, the cells were removed from the 6well plate and placed on a clean, grease-free glass slide. A fluorescent microscope was then used to study the cells (Nikon, Japan).
3. Results and discussion
3.1. Microstructure characterization
Figure 1 shows optical images of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites. Figure 1(a) shows pure Mg has a predominantly basal texture (polygrain structure) with nonuniform coarse grains. Because of its fundamental structure, the deformation of magnesium-based materials is exceedingly challenging to comprehend. Pure Mg microstructure at and along grain boundaries displayed a duplex structure consisting of light-colored alpha and dark-colored eutectic phases [65]. The existence of twinning within the grains of pure Mg could be seen. Because the strength and deterioration properties of Mg-based materials are heavily reliant on grain size, grain refining is critical. The microstructure of Mg Ni/Ti composites is shown in figure 1(b), where the inclusion of NiTi as particle reinforcement has substantially decreased the grain size of pure Mg. NiTi helps in the dispersion and strengthening of the Mg matrix composite. During dynamic recrystallization, newly refined grains form inside the twinning and around the grain boundaries of the coarser grain structure, as shown in figure 1(c) [49]. The polycrystalline microstructure of Mg 0.4Ce/ZnO2 nanocomposites in nature is seen in [50]. Nanocomposites are stable and evenly distributed across grain boundaries. The production of thin lamellar eutectic was achieved by adding nanocomposite at grain boundaries. Zinc oxide nanoparticles and cerium were used to improve the microstructure.
Figure 1. Microstructure of (a) Pure Mg (b) Mg Ni/Ti nanocomposite (c) Mg 0.4Ce/ZnO2 nanocomposites.
Download figure:
Standard image High-resolution image3.2. Hemocompatibility assay
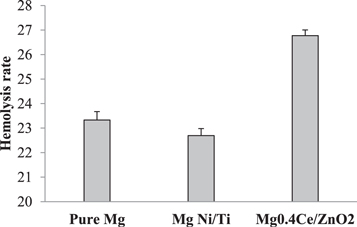
Figure 2 depicts the hemolysis rates of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites samples treated with red blood cells, which were 23.72%, 22.92%, and 26.98%, respectively. The comparison of hemolysis of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites exceeds the medical device safety standard of less than 5%. Figures 3(a)–(d) shows normal RBC cells and hemolytic activity of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites. The presence of high quantities of chlorine in the environment and conductivity to metallic corrosion causes the increase in hemolysis rate in all samples. The increase in hemolysis might be due to a pH greater than 11 surrounding magnesium samples. The erythrocyte membrane becomes unstable when the pH goes too high, resulting in erythrocyte disintegration [66]. Denatured precipitated haemoglobin is eliminated from the hemolysis study since it only evaluates the values of the supernatant, which causes lower absorbance results than the actual values. Figure 3 shows the images that are processed and analyzed in Image J software. The average cell size and count of healthy RBCs are provided in table 1. For the control, pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites, the average cell size is 0.83, 0.68, 0.70, and 0.68, respectively. Compared to Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites, pure Mg has a larger cell count of 422. The control has 287 cells, but the Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites have 293 and 393 cells, respectively.
Figure 2. Hemolysis percentage of Pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites.
Download figure:
Standard image High-resolution imageFigure 3. Hemolytic activity of red blood cells (a) control, (b) pure Mg, (c) Mg Ni/Ti and. (d) Mg 0.4Ce/ZnO2 nanocomposites.
Download figure:
Standard image High-resolution imageTable 1. Average size and count of healthy RBC cells.
| S. no | Group | Average cell size | Count |
|---|---|---|---|
| 1 | Control | 0.83 | 287 |
| 2 | Pure Mg | 0.68 | 422 |
| 3 | Mg Ni/Ti | 0.70 | 293 |
| 4 | Mg 0.4Ce/ZnO2 | 0.68 | 393 |
3.3. In-vitro degradation study
Immersion corrosion studies are the basic in-vitro procedure for examining the degradation of pure Mg, Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites. ASTM G 102–89 conducted in vitro corrosion tests in phosphate-buffered saline (PBS) at 37 °C [67]. PBS was used to immerse the samples for 3, 5, 7, and 14 days, respectively. The samples immersed in PBS degrade to produce a significant number of alkaline hydroxyl anions, Mg2+ ions and hydrogen gas and create Mg(OH)2 film, which is moderately protective according to previous literature [68]. This is because the dissolving potentials of magnesium metals are less than roughly 1.45 V at all pH settings. Several authors have proposed [69] that Mg could be dissolved as a unipositive ion (Mg+).
Dissociation of Magnesium ions
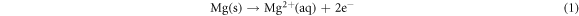
The monovalent cation will hydrolyze in water via a secondary chemical reaction spawning an excessive hydrogen gas evolution [70].
Dissociation of water
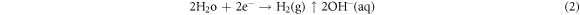
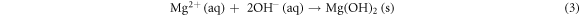
Precipitation of magnesium chloride
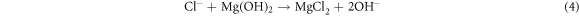
Direct formation of MgCl2
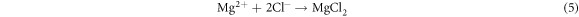
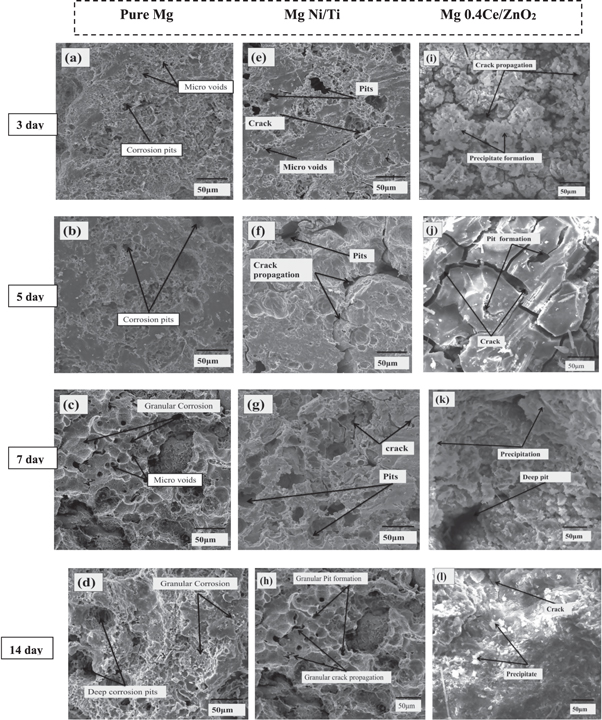
Figures 4(a)–(d) show macroscopic images of corroded pure Mg surfaces on days 3, 5, 7, and 14, respectively. From the third to the fourteenth day, the surface of pure Mg suffers from localized pitting corrosion. The protective film rupture mechanism dominated the cracking and further dissolving of the sample surfaces, and the pure Mg was more susceptible to cracking [71]. Figures 4(e)–(h) show macroscopic pictures of corroded Mg Ni/Ti surfaces on days 3, 5, 7, and 14, respectively. The protective film improves corrosion resistance by generating a barrier against ion diffusion from the phosphate buffer solution. Because the solution penetrated the surface quickly and the ions were aggressive, corrosion on the surface decreased from the third to the fourteenth day. Figures 4(i)–(l) show macroscopic images of the corroded surface of Mg 0.4Ce/ZnO2 nanocomposites on days 3, 5, 7, and 14, respectively. The protective coating serves as an ion diffusion barrier on the third and fifth days. The corrosion products in the pits on the seventh day altered the corrosion mechanism and reduced the sample size. The samples cracked along the edges, and the massive fragments crumbled and dissolved. The degradation of Mg 0.4Ce/ZnO2 nanocomposites from the edges slowly migrated inward on the fourteenth day, leaving a rough contour behind. Because of non-homogeneous degradation, the surface of Mg0.4Ce/ZnO2 began to erode from the edge and move inward, shedding pieces and losing structural integrity [72]. Mg0.4Ce/ZnO2 nanocomposites degraded much faster in PBS than pure Mg and Mg Ni/Ti nanocomposites. SEM images of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites on the 3rd, 5th, 7th, and 14th day are shown in figure 4.
Figure 4. Degradation behavior of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites in phosphate buffer solution.
Download figure:
Standard image High-resolution imageAccording to earlier literature [73], the following are the reasons for the creation of a corrosion layer on the surface: (i) When metal corrodes, Mg ions are released, resulting in a fine layer of Mg (OH)2. (ii) The new protective film layer acts as a local barrier, keeping ions from reaching the surface. (iii) A multitude of protective layers shield the material from sensitive Cl− environments. Figures 5(a)–(c) shows the corrosion topography of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites surfaces immersed in PBS on the third day. In a static environment, the corrosion mechanism causes additional surface corrosion products to be deposited. The product of corrosion is mainly magnesium hydroxide, which forms a defensive coating on the surface and produces hydrogen as a by-product [74]. On the third day, figure 5(a) shows white breakdown products spread across the whole surface of pure Mg. The surface was also originally free of cracks, which reduced Cl2 ion penetration into the new surface. Large pits developed small pits from the surface of Mg Ni/Ti nanocomposites, as revealed in figure 5(b), indicating persistent, localized corrosion. It is nearly uniform, with some localized pits. As a protective layer, white corrosion products are scattered more erratically on Mg 0.4Ce/ZnO2 nanocomposite surfaces, as seen in figure 5(c). The protective layer was dense, rough, and heterogeneous, forming good resistance to corrosion. The stability of the protective film is affected by continuous interaction with PBS, which is vital for corrosion resistance. On the fifth day, the corrosion pits emerged over the entire surface of pure Mg by breaking the protective film, as shown in figure 5(d). Now with massive pits, severe localized corrosion is readily obvious. Figure 5(e) shows the surface of Mg Ni/Ti having adjacent large pits with new crack spots. The disintegration of the old protective film attacks the fresh surface. This would result in more localized corrosion at the crack site, and the protective film would be easily broken. Figure 5(f) shows the surface of Mg 0.4Ce/ZnO2 nanocomposites granular corrosion occurs with more localized pits at the crack site by breaking the protective film. This is because aggressive chloride ions penetrate these protective layers to reach the new surface. During the fifth day, the size of the specimen remains unchanged, but the corrosion mechanism for Mg 0.4Ce/ZnO2 nanocomposites is more severe than for pure Mg and Mg Ni/Ti nanocomposites. Because of the constant interaction with PBS, the entire surface begins to erode as the immersion time increases. Rajan et al, 2022 reported that the Mg-coated specimens' bioactivity in SBF immersion demonstrated apatite production in 5 days [69]. The white layer that grew on the surface of the samples remained unchanged after the seventh day. The PBS-exposed protective layer had penetrated the top layer and reached the new surface. As seen in figures 5(g), (h), deep pits have grown from the new surface of pure Mg and Mg Ni/Ti nanocomposites, causing further degradation. It was seen that both surfaces were covered with a larger and interconnected deep pit. Over time, the pits grew wider and connected to nearby pits, causing the old surface layer to fall off. Figure 5(i) shows that the surface of Mg 0.4Ce/ZnO2 nanocomposites is deeply corroded due to pit growth, and new degradation products were formed in the pits. Pitting degradation and pit enlargement is part of the corrosion process, and pitting degradation reoccurs at the newly exposed surfaces. Though the newly formed degradation product and deposited white layer protected the sample from rapid degradation and reduced the corrosion, the sample degradation could not be completely prevented, as evidenced by the change in the size of samples immersed in PBS. On the fourteenth day, as shown in figure 5(j), pure Mg was susceptible to localized corrosion caused by corrosive ions permeating the surfaces through pits. The protective layer on top acts as an ion barrier; such aggressive ions like Cl− can permeate it over time, producing localized corrosion and surface breakdown. Mg Ni/Ti nanocomposite surface thin film develops discontinuously at its initial stage, forming a local barrier and providing instant corrosion resistance, as shown in figure 5(k). This is because the presence of Ti and Ni acts as a barrier, causing the material to degrade at a slower rate. Figure 5(l) shows the structural stability of the Mg 0.4Ce/ZnO2 nanocomposites is weakened by their lower corrosion resistance. Corrosion products in pits become denser and break down when the immersion time is increased. The discharge of surface fragments may have been regulated by the corrosion products, which may have protected the surface beneath it and so limited localized corrosion development [75].
Figure 5. SEM images of pure Mg, Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites tested in Phosphate buffer solution.
Download figure:
Standard image High-resolution imageAs the spread of pitting corrosion got more brutal to keep the entire degradation layer, the surface eventually fractured into shards. When the protective layer was dissolved by targeting the new surface, pure Mg was shown to have more interconnected deep pits. The presence of Ni and Ti on the Mg Ni/Ti nanocomposite surface safeguarded it from severe corrosion. Little pits are visible on the surface of the Mg Ni/Ti nanocomposite. Mg 0.4Ce/ZnO2 nanocomposites structural stability deteriorates after 14 days when compared to pure Mg and Mg Ni/Ti nanocomposites. A deterioration of the basal texture and the existence of secondary phases are thought to contribute to more weight loss. According to previous studies, a more uniform corrosion rate depends on a homogeneous second-phase distribution and a refined matrix free of cracks. The mechanism of corrosion is heavily influenced by surface microstructure and local pH. The health of humans is jeopardized by rare elements.
The yield strength of pure Mg was just about half the strength of natural bone, and it was not approved for long-term use (110 MPa). Selection of alloys and mechanical processing must be included to enhance yield strength for implant applications. On the other hand, the material's deterioration rate must be changed based on the fracture type and expected healing time. Metallic implants must have strong corrosion resistance to be utilized successfully in the clinic since severe corrosion reduces mechanical strength and creates harmful ion release. The discharge of harmful metal ions or particles into the body during corrosion is another disadvantage of commercial implants. They must remain as permanent implants (plate, screw, and pin) inside the human body until natural tissue has fully recovered.
3.4. Cytotoxicity assessment
To assess the possibility of the safe use of nanocomposites in orthopaedic applications, cytotoxic studies were performed. Cytotoxicity was assessed by MTT assay. Figure 6 shows the percentage of cell viability of MG63 osteoblast cells incubated in Mg and its nanocomposites after 24 h of incubation. In the present study, the % cell viability of pure Mg was observed as 235.64%, and it was high when compared to other Mg nanocomposites. This might be due to better cell adhesion by more cell spreading and a greater degree of cell attachment [76]. The % viability of Mg 0.4Ce/ZnO2 nanocomposite was found to be 165.03%, and it is supported by the previous report stated that the Mg Zn/CeO2 nanocomposites exhibited no cytotoxicity to MC3T3-E1 pre-osteoblast cells and based on this findings, the feasibility of Mg Zn/CeO2 nanocomposites for use as orthopedic implants is systematically discussed [77].
Figure 6. Effect of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites on cell viability.
Download figure:
Standard image High-resolution imageAnother report stated that ZnO nanoparticles in concentrations up to 20 mg/l have no adverse effect on HeLa cells [78]. Our previous findings demonstrated the results of the MTT assay for the Mg 0.4Ce/ZnO2 surface appear to have a cytotoxicity effect after one day due to an increase in pH [71]. The % viability of Mg Ni/Ti nanocomposite was found to be 136.18%. Our results, evidenced by the previous report by Tiong Hou Damien Ong et al, 2017 suggested that Mg 2.5 vol% TiO2, Mg TiC, and Mg TiN have the potential to be qualified as orthopedic implant materials subject to the next level of cytotoxicity tests and results of in vivo studies [79]. Jana Markhoff et al, 2017. The osteoblastic cell line MG-63 as well as human osteoblasts, fibroblasts, and macrophages, were cultured on four titanium alloys to verify cell-specific biocompatibility. Thereby, Ti6Al4V SLM and NiTi revealed the best results regarding the metabolic activity. After 24 h, all the investigated materials showed high cell viability (>90%) in the present study. Based on the ISO 10993–5:2009 standard, the MTT assays have no cytotoxicity potential if the viability is more significant than 70% of the negative control. This indicates that both pure Mg and its nanocomposites are biosafe in terms of cellular metabolism.
3.5. Biomineralization by alizarin red staining (ARS)
Figures 7 (a)–(c) presents the in vitro osteogenic activity of MG63 cells on Mg 0.4Ce/ZnO2 and Mg Ni/Ti nanocomposites with pure Mg surfaces. The calcium deposition appears as a red brick dot on the surface of Mg 0.4Ce/ZnO2 nanocomposites and Mg Ni/Ti. When cells are grown directly with a material, environmental changes such as the shift of pH, release of ions, and evolution of hydrogen were discovered to be particularly sensitive. The initial chronological events in contact with a material surface are adhesion, spreading, and cell migration, which are critical for cell survival. Only cells grown in Mg nanocomposites had red biomineralized nodules, which correspond to a calcium-rich extracellular matrix, as shown in figures 7(a) and (b). Pure Mg surface showed less or an absence of calcium deposition by MG63 cells in figure 7(c). Increases in alkaline phosphatase (ALP), frequently followed by an increase in bone mineralization, are linked to calcium deposits [80]. After 24 h, the MG63 cells appear uniformly spread over the sample surface in this investigation.
Figure 7. Cell-material interaction of MG 63 cells with surface of (a) Mg 0.4Ce/ZnO2, (b) Mg Ni/Ti, and (c) pure Mg nanocomposites after 24 h.
Download figure:
Standard image High-resolution image4. Conclusions
- The results of in-vitro corrosion show progressive localized corrosion on pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites. Mg 0.4Ce/ZnO2 nanocomposites exhibited severe corrosion from the seventh to the fourteenth day, and the surface edges started to erode inward direction.
- The hemocompatibility results showed higher hemolysis in pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites, greater than safer medical device values (>5).
- From the cytotoxicity results, the percentage of cell viability of MG63 osteoblast cells was higher in pure Mg than in Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites.
- Biomineralization results showed more calcium deposition by MG63 cells on the surface of Mg Ni/Ti and Mg 0.4Ce/ZnO2 nanocomposites than pure Mg. Pure Mg surface showed less or an absence of calcium deposition by MG63 cells.
- Hence, the present study's finding suggested that combining Mg nanocomposites could provide new avenues to the medical development of orthopaedic implant applications.
Acknowledgments
The National University of Singapore's Associate Professor and Department of Mechanical Engineering, Dr Manoj Gupta, is acknowledged by the authors for kindly providing the sample for the study.
Data availability statement
The data cannot be made publicly available upon publication because no suitable repository exists for hosting data in this field of study. The data that support the findings of this study are available upon reasonable request from the authors.
Declarations
Conflict of interest: there is no conflict of interest.