Abstract
Ionic liquids are promising candidates with huge potential in combination with thermosetting polymers. However, the behavior of ionic liquid modified epoxy system towards outdoor environmental conditions are seldom reported. Therefore, it is interesting to study the effect of accelerated weathering on ionic liquid modified bioepoxy blends. In this work, bio-based epoxy resin and 1-Butyl-3-methylimidazolium chloride (BMIM[Cl]) ionic liquid blends were prepared by melt mixing method. The concentration of ionic liquid used was 0, 5, 10, 20, and 30 phr. The miscibility, morphology, thermo-mechanical, and surface hydrophilicity of the ionic liquid modified bioepoxy blends were studied before and after the accelerated weathering test. The miscibility of the blends was studied by Fourier transform infrared spectroscopy (FTIR). The FTIR results demonstrate the presence of charge transfer complexation reaction between ionic liquid and bioepoxy resin. The tensile strength and modulus were reduced while the elongation at break is increased with the addition of ionic liquid. On the other hand, the glass transition temperature and thermal stability of the bioepoxy blends were reduced with the addition of ionic liquid. The contact angle value increases with the incorporation of up to 10 phr ionic liquid, this is followed by a decrease. The interaction between the ionic liquid and bioepoxy resin has vanished after the weathering test. The elongation at break was reduced dramatically after the weathering test, especially for 20 and 30 phr blends. The thermal stability of the weathered samples is similar to that of the samples before weathering. While the Tg values of the blends are stable with respect to neat bioepoxy resin. On the other hand, the contact angle value of the bioepoxy blends increased after the weathering test due to the increased surface roughness after weathering.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Ionic liquids are liquid electrolytes comprising of ionic-covalent crystalline structures, which are classified as molten salt at room temperature [1, 2]. Their distinct properties such as non-volatility, high thermal and chemical stability, inflammability, and ionic conductivity find suitable for numerous applications [3–5]. Besides, they are widely accepted as catalyst/curing agents, lubricants, plasticizers, antistatic agents, thermal material, magnetic material, intelligent material, gas absorption material, etc [6]. Ionic liquids report some excellent applications when combined with thermosetting polymers like epoxy resin. For instance, the modification of epoxy resin with ionic liquid has shown excellent mechanical properties, thermal stability, and adhesive properties [7–9].
The ionic liquid has reportedly proved to exhibit different behavior depending on its amount in epoxy resin [9]. At lower ionic liquid content, it acts as a catalyst or crosslinking agent for epoxy resin [10, 11]. The usefulness of ionic liquid as a crosslinking agent is possible either by mixing alone with epoxy resin or in ratios with any other conventional curing agents [12, 13]. Such crosslinking is possible without compromising the thermal stability, hydrophobicity, and thermomechanical properties of epoxy resin [14–17]. Nguyen et al [17] investigated the influence of phosphonium based ionic liquids combined with phosphonate, carboxylate, and phosphate counter anions as a new route for the design of epoxy resin networks. The studies have shown that the addition of a small amount of ionic liquid (10, 20, and 30 phr) leads to the formation of networks with improvement in thermal, mechanical (flexural strength and fracture toughness) and hydrophobic properties. Ostrowska et al [18] studied a few of ammonium ionic liquids as support for palladium complex catalyst in order to cure the epoxy resin. The ammonium ionic liquids used for crosslinking epoxy resin were didecyldimethylammonium tetrafluoroborate [d2m2am][BF4], 1-methyltheobromine tetrafluoroborate [mthb][BF4], didecyldimethylammonium theobrominate [d2m2am][thb] and didecyldimethylammonium theophyllinate [d2m2am][thp]. The authors conclude that the type of ionic liquid used to cure epoxy resin can greatly affect the catalytic properties of epoxide-supported palladium catalyst.
At higher content, the ionic liquid acts as lubricant and plasticizer [19–24]. Sanes et al [25] studied the tribological behavior of 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid modified epoxy resin. It was found that the ionic liquid in epoxy resin improves internal lubrication. Thus, reducing the friction and wear rates of the epoxy resin. Similarly, Avilés et al [26] reported that the addition of tri-[bis(2-hydroxyethyl)ammonium)] citrate ionic liquid to epoxy resin improved the self-lubrication and wear-resistant properties. It was found that ca. 50% reduction in friction coefficient was achieved for ionic liquid modified epoxy resin due to the release of the ionic liquid lubricant under load. Besides, the incorporation of ionic liquid was found to exhibit room temperature flexibility in epoxy resin. In an interesting work, Hameed et al [27] prepared flexible epoxy composites using ionic liquids that behaved like ductile thermoplastics. The composite prepared exhibited excellent transparency and room temperature flexibility, similar to thermoplastic materials. Soares et al [28] studied the effects of different ionic liquids modified epoxy networks cured with 4,4'-methylene-bis 3-chloro-2,6-diethylaniline (MCDEA). They found that the presence of the ionic liquids resulted in a decrease in the glass transition temperature (Tg) of epoxy resin. This is due to the plasticizing effect of ionic liquid present at the networks of cured epoxy resin.
Even though ionic liquid finds enormous applications with epoxy resin, their accelerated weathering studies are seldom reported. It is important to study the effect of harsh outdoor exposure on such materials. Moreover, the reported works are all on nonbiodegradable epoxy resins. The aim of this work is the fabrication of bio-based epoxy/ionic liquid blends. The bioepoxy blends containing 5, 10, 20 and 30 phr ionic liquid were prepared. The prepared blends before weathering and after 240 h (10 days) of weathering were systematically studied for clear understanding. An attempt has been made for precise comparison of physical, thermal, mechanical, morphological, and spectroscopic studies of the blends before and after weathering. This paper will also give an insight into the outdoor applications of ionic liquid modified bioepoxy blend.
Materials and methods
Materials
Bio-based epoxy resin (SR Greenpoxy 56) and its curing agent (SD surf clear) used in this study were supplied by Cobra International Co Ltd, Chonburi, Thailand. The bioepoxy resin has 56% of its epoxy structure derived from plant origin and has a density of 1.198 gm/cc and a viscosity of 800 mPa·s at 25 °C. The imidazolium-based ionic liquid, 1-Butyl-3-methylimidazolium chloride (BMIM[Cl]), was purchased from Sigma Aldrich. The BMIM[Cl] is a high-purity ionic liquid ≥99.0% with low water content. The melting point of BMIM[Cl] ionic liquid is 70 °C.
Sample preparation
The samples of bioepoxy/ionic liquid blends were prepared by melt mixing method. Initially, the epoxy resin was mixed with the ionic liquid in a glass beaker at 80 °C using a magnetic stirrer. After the homogenous solution of bioepoxy/ionic liquid mixture was obtained, the temperature was reduced to room temperature. Finally, the hardener was poured into the bioepoxy/ionic liquid mixture in stoichiometric amounts (100:37) and mixed until a homogenous solution was obtained. The homogenous mixture was then poured into a Teflon coated mold and cured for 24 h at room temperature followed by post-curing at 60 °C for 8 h. The samples were coded as EIL5, EIL10, EIL20, and EIL30 for 5, 10, 20 and 30 phr of ionic liquid modified bioepoxy blends, respectively.
Characterization techniques
Fourier transform infrared spectroscopy (FTIR)
Perkin Elmer spectrum 2000 FTIR spectroscopy was used to study the interaction existing between the ionic liquid and bioepoxy resin. The spectra were measured by scanning in the range of 4000 to 400 cm−1.
Mechanical properties
Testometric M500-25AT, a universal testing machine (UTM), was used to measure the tensile properties of the samples before weathering and after weathering. The samples were tested according to ASTM D 638 with sample dimensions of 100 × 10 × 3 mm3. A load cell of 100 N was used for testing. All the samples were fixed vertically between the grips of the machine at a gauge length of 50 mm, and the test was conducted with a crosshead speed of 5 mm min−1. At least 5 samples before weathering and after weathering were used to measure and compare the tensile properties.
Scanning electron microscope (SEM)
Scanning electron microscope FEI Quanta 450 in low vacuum mode at 10kV was used to investigate the structural changes before and after weathering.
Differential scanning calorimetry
DSC Q2000 (TA instruments) differential scanning calorimetry was used to measure the Tg of the blends in the N2 atmosphere. The samples of ca. 5 mg were cooled down to start temperature (−50 °C) followed by isothermal for 3 min to remove any thermal history. Later, the samples were heated from −50 to 200 °C at a rate of 10 °C min−1. The samples were then cooled to −50 °C at a rate of 20 °C min−1 and heated again from −50 to 200 °C under the same condition. The second heating cycle was selected to plot DSC curves.
Thermal degradation behavior
Thermogravimetric analyzer TGA/DSC 3 + HT/1600 (Mettler Toledo Ltd), was used to measure the thermal degradation behavior of the ionic liquid modified bioepoxy blends. Approximately 6 mg of the samples was placed in a ceramic pan and scanned in a temperature range of 30 to 800 °C at a heating rate of 10 °C min−1. The change in mass with respect to temperature was determined and plotted.
Contact angle measurements
The surface properties of the blends were measured using the data physics-contact angle system OCA supplied from LMS instruments. For contact angle measurements, ca. 6 μl distilled water was placed as a sessile drop on the sample surface, and the corresponding contact angle was monitored. At least 10 measurements were taken to determine the wettability of the samples.
Accelerated weathering tests
Accelerated weathering tests were carried out in the Q-Sun Xenon test chamber (model Xe-3) Q-Lab Corporation, USA. The tests were performed as per ASTM standard G 155-13 (cycle 1) using a daylight filter [29]. The samples were placed inside the weathering chamber for a total period of 240 h at 0.35 W m−2 irradiance and 340 nm wavelength. The exposure cycles followed in our tests were 1.42 h light at 63 °C black panel temperature and 0.18 h in light and water spray.
Results and discussion
The visual appearance of the samples before and after the weathering test
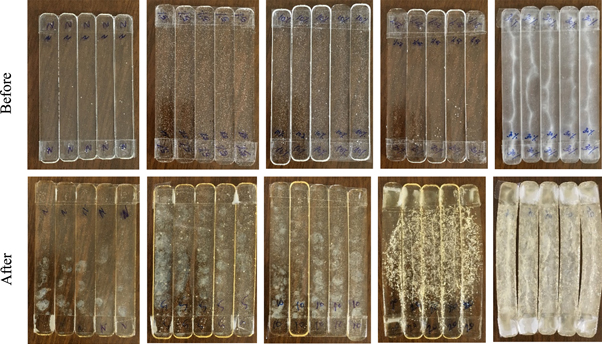
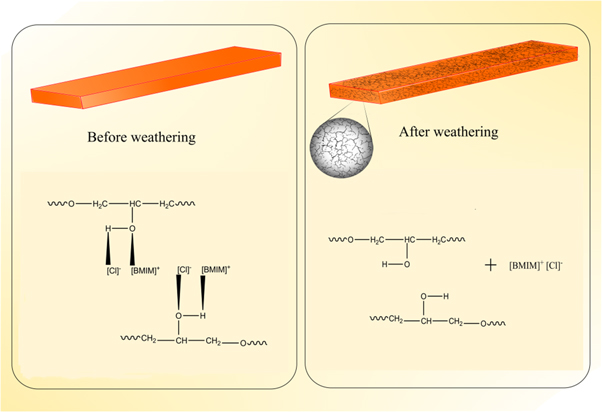
The photographs of bioepoxy/ionic liquid blends before and after the weathering test are shown in figure 1. From the figure, it can be seen that up to 20 phr ionic liquid, the system is entirely transparent. Thus, it can be argued that the ionic liquid has very strong interactions with bioepoxy resin. It may be due to charge transfer complexation reaction, i.e., electron donor-electron acceptor complex reaction between oxygen and hydrogen atoms of epoxy hydroxyl groups and charged species of ionic liquid [30]. However, at 30 phr, the blends are translucent. Note that during mixing, the blend samples were all transparent in the molten state, indicating the microscopic homogeneity of the mixture. The blends after weathering are also transparent, but a large number of cracks are generated in the polymer sample especially at higher concentrations of the ionic liquid. This is because the interaction between ionic liquid and bioepoxy resin may be broken during weathering, and it leads to breakage and hence, cracks. The schematic representation of the same is depicted in scheme
Figure 1. Photographs of bioepoxy/ionic liquid samples (Neat, EIL5, EIL10, EIL20, and EIL30) before and after weathering.
Download figure:
Standard image High-resolution imageScheme 1. The schematic of bioepoxy/ionic liquid samples before and after weathering.
Download figure:
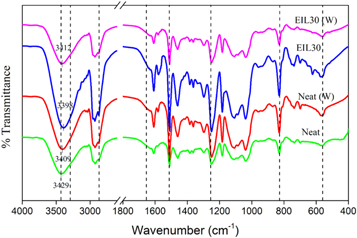
Standard image High-resolution imageThe FTIR spectra of the ionic liquid modified bioepoxy blends before weathering and after weathering has been taken. As shown in figure 2, it is evident from the spectra that the bioepoxy/ionic liquid samples undergo stretching of the hydroxyl group [27]. A slight shift of the bands of hydroxyl functional groups to lower wavelength (3429 to 3398 cm−1) suggests the possible interaction between free hydroxyl groups of bioepoxy chains and the charged species of the ionic liquid. The redshift indicates the charge transfer complexation between OH groups and BMIM+ and Cl− ions, and these interactions are stronger than self-associated hydrogen bonding. Therefore, the ionic liquid is miscible with bioepoxy systems. The bands associated with other functional groups remain in the same position. On the other hand, the hydroxyl functional groups of the samples after weathering did not show any redshift. That means there is no charge transfer complexation reaction between ionic liquid and bioepoxy resin after the weathering test. Thus, it can be observed that the interaction between ionic liquid and bioepoxy resin is completely lost after the weathering test. It should be noted that the other bands associated with other functional groups also remain unaffected.
Figure 2. FTIR spectra of bioepoxy/ionic liquid blends before and after weathering.
Download figure:
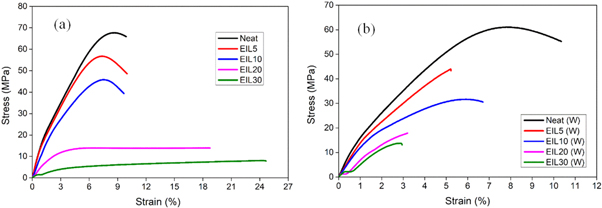
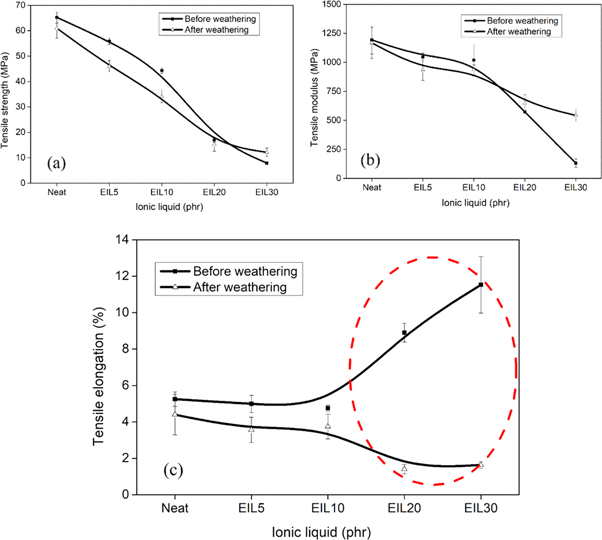
Standard image High-resolution imageThe tensile properties of the blends before weathering and after weathering were investigated, and the results are shown in figures 3 and 4. The tensile strength, modulus, and elongation at break of each sample are compiled in table 1. The tensile strength of the blends before and after the weathering test is shown in figure 4(a). The figure shows a decrease in tensile strength with the addition of ionic liquid irrespective of weathering. This is because the ionic liquid is a very low strength material that acts as a lubricant or plasticizer in the bioepoxy matrix, and therefore, the crosslink density is reduced and hence the tensile strength decreases. The reduction in mechanical properties especially at higher loadings of the ionic liquid is caused by the high degree of plasticization of ionic liquid in the epoxy matrix [31]. The samples after weathering have slightly lower tensile strength values when compared with samples before weathering. This is expected because after weathering cracks are generated in the sample, and these cracks act as nucleating sites for additional fracture. The tensile modulus of the blends before and after weathering is shown in figure 4(b). Up to 10 phr ionic liquid, the tensile modulus of the samples before and after weathering remain more or less stable. However, at high concentrations, the value drops. The weathering may increase the internal friction and hence reduces the segmental mobility of polymer chains. Therefore, these samples show higher modulus values compared with samples before weathering [32]. The tensile elongation of the blends is shown in figure 4(c). From the figure, up to 10 phr, the tensile elongation of ionic liquid modified bioepoxy samples remains more or less the same. This is followed by a rapid increase in the tensile elongation. This means that the ionic liquid acts as a suitable plasticizer at approximately or above 10 phr. It should be noted that samples after weathering show very poor tensile elongation, especially at 20 phr and 30 phr ionic liquid. This is due to the formation of cracks that are generated during the accelerated weathering test.
Figure 3. Stress/strain curve of ionic liquid modified bioepoxy resin (a) before weathering and (b) after weathering.
Download figure:
Standard image High-resolution imageFigure 4. Comparison of mechanical properties of ionic liquid modified bioepoxy blends before and after weathering (a) tensile strength (b) tensile modulus and (c) tensile elongation.
Download figure:
Standard image High-resolution imageTable 1. Tensile properties of ionic liquid modified bioepoxy blends.
| Before weathering | After weathering | |||||
|---|---|---|---|---|---|---|
| Blends | Tensile strength (MPa) | Elongation at break (%) | Tensile modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) | Tensile modulus (MPa) |
| Neat | 65.2 ± 2.2 | 5.3 ± 0.4 | 1193.3 ± 121.7 | 61 ± 3.9 | 4.4 ± 1.1 | 1168 ± 134.0 |
| EIL5 | 55.8 ± 1.3 | 5 ± 0.5 | 1046.5 ± 34.1 | 46 ± 2.3 | 3.6 ± 0.7 | 937 ± 91.4 |
| EIL10 | 44.3 ± 1.1 | 4.8 ± 0.2 | 1019.6 ± 136.5 | 34.1 ± 2.5 | 3.8 ± 0.7 | 934.3 ± 43 |
| EIL20 | 17 ± 0.8 | 8.9 ± 0.5 | 572 ± 9.9 | 15.3 ± 2.7 | 1.4 ± 0.3 | 649.9 ± 72.1 |
| EIL30 | 7.9 ± 0.6 | 11.5 ± 1.6 | 131.3 ± 35.7 | 12.2 ± 1.7 | 1.6 ± 0.2 | 542.7 ± 55 |
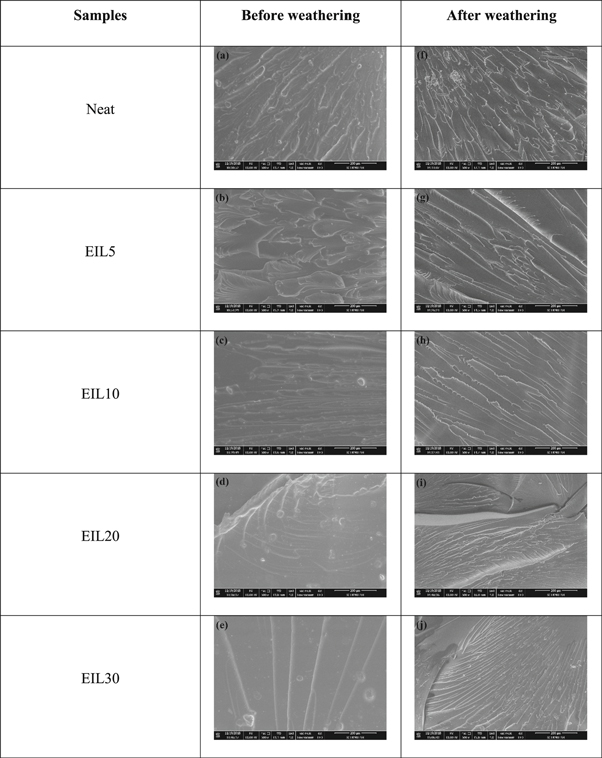
The SEM micrographs of the fractured bioepoxy samples after tensile measurements were taken before and after accelerated weathering and are shown in figure 5. The SEM micrographs before weathering show typical brittle fractures. However, the number of fractures decreases with the ionic liquid. Very few fractures were observed for 30 phr ionic liquid modified bioepoxy blends. This is due to the rubbery nature of the sample at higher ionic liquid content. Once the sample becomes rubbery, the typical epoxy fracture surface disappears.
Figure 5. SEM image of the fracture surface of ionic liquid modified bioepoxy samples.
Download figure:
Standard image High-resolution imageOn the other hand, the bioepoxy blends after weathering show typical brittle fracture irrespective of the ionic liquid content. Thus, the morphology of weathered samples represents weak interaction between ionic liquid and bioepoxy resin after the weathering test. In other words, the weathered samples are more brittle and with behavior closer to a neat bioepoxy thermoset.
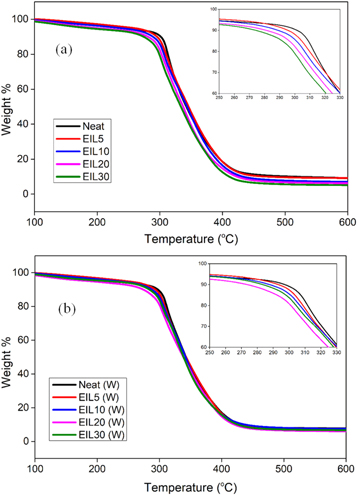
The thermal degradation behavior of bioepoxy blends is shown in figure 6. The initial degradation temperature (IDT), maximum degradation temperature (MDT), and final degradation temperature (FDT) obtained from the thermogram are listed in table 2. From the table, the IDT, MDT, and FDT of the bioepoxy blends gradually decrease with increasing ionic liquid content. It implies that the presence of ionic liquid lowers the degradation temperature of the bioepoxy blends. The decrease in thermal stability can be explained based on the following reasons. The first reason could be the comparatively lower degradation stability of the ionic liquid. The second reason is due to the reduced crosslink density of the blends because of the charge transfer complexation reaction and confinement of bulky ionic liquid molecules within the bioepoxy thermoset. On the other hand, the IDT, MDT, and FDT are not much affected by accelerated weathering. That means the weathering studies does not have any effect on the thermal stability of the bioepoxy blends.
Figure 6. Thermal degradation behavior of ionic liquid modified bioepoxy resin (a) before weathering and (b) after weathering.
Download figure:
Standard image High-resolution imageTable 2. The IDT, MDT, and FDT of ionic liquid modified bioepoxy blends before and after weathering.
| IDT | MDT | FDT | ||||
|---|---|---|---|---|---|---|
| Blends | Before weathering | After weathering | Before weathering | After weathering | Before weathering | After weathering |
| Neat | 300.27 | 299.25 | 311.45 | 311.44 | 410.30 | 409.08 |
| EIL5 | 295.79 | 294.76 | 309.07 | 310.36 | 411.31 | 408.06 |
| EIL10 | 292.32 | 291.30 | 307.78 | 311.43 | 403.57 | 402.34 |
| EIL20 | 290.07 | 286.80 | 306.71 | 304.34 | 400.57 | 401.1 |
| EIL30 | 284.56 | 287.82 | 304.34 | 305.42 | 390.91 | 400.10 |
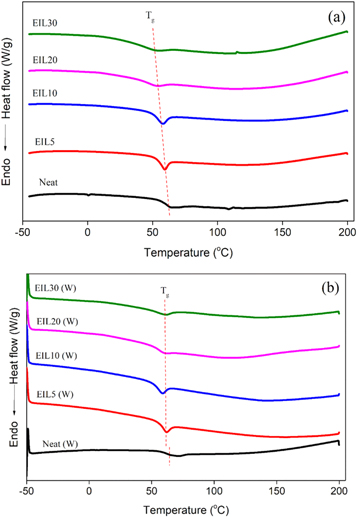
The DSC studies of ionic liquid modified bioepoxy blends were investigated. Figures 7(a)–(b) shows the second DSC heating scans of bioepoxy/ionic liquid blend before and after weathering, respectively. From the DSC thermograms, the blends before weathering show a constant decrease in Tg of the epoxy phase with the addition of ionic liquid. The decrease in Tg is due to the favourable charge transfer complexation reaction between ionic liquid and bioepoxy thermoset. The charge transfer complexation reaction between ionic liquid and bioepoxy resin is stronger at higher concentrations of ionic liquid, this will indeed reduce the crosslink density and Tg of the epoxy blends. The Tg observed at 55 °C for the neat bioepoxy system is reduced to 45 °C with the addition of 30 phr ionic liquid. However, the samples after weathering did not show such a change. Only a marginal decrease of 2 to 3 °C was observed with the addition of ionic liquid. This is because the interaction between ionic liquid and bioepoxy resin may be broken, leading to the degradation of the ionic liquid during weathering. Therefore, the plasticizing action of ionic liquid in the bioepoxy blends is lost, and hence Tg shows little variations. The marginal drop in Tg is due to the ionic liquid remaining dissolved in the bioepoxy blends.
Figure 7. DSC thermograms of ionic liquid modified bioepoxy resin (a) before weathering and (b) after weathering.
Download figure:
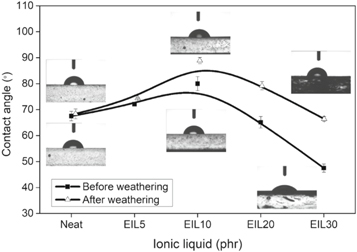
Standard image High-resolution imageThe water contact angle values before and after weathering are shown in figure 8. The contact angle values of all the blends studied are less than 90 °. This implies that the blend samples before and after weathering are hydrophilic. However, there is an increase in the contact angle value from 67.5 (neat bioepoxy) to 80 ° for 10 phr ionic liquid modified bioepoxy blends. This might be due to the confinement of ionic liquid within the networks of epoxy structures. Nevertheless, the contact angle decreased to 47.5, with a further increase in the ionic liquid above 10 phr. This is because BMIM[Cl] based ionic liquid reported showing more hydrophilic behaviour with polymer blends [33]. In other words, at higher concentrations, ionic liquid migrates to the surface of samples, and hence the contact angle decreases [34]. This is because the hydroxyl groups present in the ionic liquid interact with water droplets. On the other hand, the samples after the weathering test show high contact angle values may be due to the increased roughness of the sample surface after the weathering test. The increased roughness of samples is due to the generation of cracks at the surface of the sample during the weathering test. The work of adhesion (Wa) and the spreading coefficient (Sc) of the blends are calculated and are listed in table 3. The Wa is the amount of work needed to separate the water droplet from the blend surface. While the Sc implies the spreading of the liquid over the blend surface [35]. The high value of Wa and Sc for 30 phr ionic liquid modified bioepoxy blend (before weathering) indicates the spontaneous spreading of water droplet over the blend surface.
Figure 8. Comparison of contact angle values of ionic liquid modified bioepoxy resin.
Download figure:
Standard image High-resolution imageTable 3. Contact angle, work of adhesion and spreading coefficient of neat bioepoxy, EIL5, EIL10, EIL20, and EIL30.
| Contact angle (°) | Work of adhesion (Wa) | Spreading coefficient (Sc) | ||||
|---|---|---|---|---|---|---|
| Samples | Before | After | Before | After | Before | After |
| Neat | 67.5 ± 1.8 | 68.4 ± 1.9 | 100.66 | 99.55 | −44.94 | −46.05 |
| EIL5 | 72.1 ± 0.6 | 74.4 ± 0.2 | 95.24 | 92.43 | −50.36 | −53.17 |
| EIL10 | 80.0 ± 2.7 | 88.9 ± 1.2 | 85.44 | 74.25 | −60.16 | −71.35 |
| EIL20 | 65.1 ± 2.2 | 79.2 ± 1.6 | 103.42 | 86.47 | −42.18 | −59.13 |
| EIL30 | 47.5 ± 1.6 | 66.4 ± 0.9 | 121.93 | 102.00 | −23.67 | −43.60 |
Conclusion
The accelerated weathering studies of ionic liquid modified bioepoxy samples were carefully analyzed. The higher concentration of ionic liquid noticeably shows different behavior in weathering conditions. At 20 phr and 30 phr ionic liquid content, the exposure of 240 h of accelerated weathering in the controlled chamber tends to deteriorate ionic liquid from the bioepoxy matrix. This is because of the breaking of ionic interaction between the ionic group and the hydroxyl group of bioepoxy resin. This is also evident from the thermal, mechanical, and morphological studies. The morphology of tensile fracture surface of these blends shows a typical brittle behavior confirmed by SEM analysis. On the other hand, the elongation at break drastically is reduced, while the Tg of the bioepoxy thermoset was not affected after the weathering test these results signifying the poor interaction between the ionic liquid with bioepoxy resin after the weathering test. The FTIR studies revealed the formation of the complexation reaction between the ionic group and bioepoxy resins. While after weathering, the absence of any redshift proves the dissociation of complexation reaction between ionic groups of ionic liquid and OH groups of bioepoxy resin. Furthermore, at lower concentrations of ionic liquid, water contact angle remains unchanged due to confinement of ionic liquid within the networks of epoxy. While at higher concentrations, the samples become more hydrophilic due to the presence of more ionic liquid on the surface of samples. However, the water contact angle values show marginal variations after weathering shows the absence of ionic liquid on the surfaces. Thus, it can conclude that accelerated weathering of ionic liquid modified bioepoxy resin undergo deterioration, and it resulted in crack formation with in the material.
Acknowledgments
This research was funded by King Mongkut's University of Technology North Bangkok. Contract number: KMUTNB-63-KNOW-002.