Abstract
The development of reliable and environmentally benign electrochemical sensor for the detection of carcinogenic As(III) is of great scientific and public interest. Herein, thin film of novel Fe2V4O13-polypyrrole nanocomposite was prepared on the screen printed electrode (SPE) and thus the prepared nanocomposite modified SPE was successfully used as one shot disposable electrochemical sensor for the detection As(III). The proposed sensor has high sensitivity towards As(III), wherein it exhibits wide linear range, from 0 to 500 ppb, with the detection limit (LOD) of 0.3 ppb, which is well below the value prescribed by World Health Organization (WHO). Therefore, the proposed sensor could be used for the detection of As(III) present in real samples.
Export citation and abstract BibTeX RIS
1. Introduction
The toxic and carcinogenic arsenic including As(III) and As(V) is widely distributed in the Earth's crust and thereby contaminate the ground water. Further, the use of arsenic in the manufacture of fertilizers, herbicides, insecticides and semiconductor industries lead to the contamination of surface water [1]. Thus, the contamination of arsenic in drinking water has been found in many parts of the world [2]. Arsenic is mainly exists in As(III) and As(V) wherein the solubility of As(III) is high and ∼50 times more toxic than the As(V). The long term exposure to the arsenic causes skin, bladder and lung cancer, dermal changes, cardiovascular, gastrointestinal, genotoxic, mutagenic and carcinogenic diseases [3]. As a result, tremendous research effort has been put forward in the past decade to detect/quantify arsenic in real matrices.
For instance, Nadeau et al, successfully developed a colorimetric based simple tool kit for the detection of arsenic [4] for field applications. However, the proposed method produces extremely toxic arsine gas and it is not reliable for low concentrations (<10 ppb). Contrary, electrochemical quantification using stripping voltammetry is extensively adopted owing to its excellent sensitivity and uniqueness in the detection of elements in trace level (ppb level) where noble metals based gold and platinum electrodes have been used [5–7]. However, these reported electrodes usually associated with the drawback of low electron transfer kinetics, limited sensitivity and reproducibility. Therefore, the development of reliable and highly sensitive with reproducible protocol is necessary for on-field application.
Screen-printed electrodes (SPE) can be used as single-shot disposable electrodes for the detection of As(III) as SPEs can be produced cheaply on large scale production and simply be thrown away after the use. Recently, Fe3O4-ionic liquid composite modified SPE has been proposed to detect As(III) [8]. However, the preparation of Fe3O4 composite using ionic liquid is complex/tedious and expensive.
Contrary, the electrochemical sensors reported for the detection of arsenic mostly uses acid electrolytes and therefore it is prime requisite that the electrode modifier must be insoluble in acid electrolytes. Most of the inorganic electrode modifiers are soluble in acid electrolyte and thus not many reports are available in the literature. As we know, polypyrrole in not soluble in acid electrolytes and Fe2V4O13 is highly electro-active. Thus, the Fe2V4O13-polypyrrole nano-composite has been prepared by a simple wet chemical method. The prepared Fe2V4O13-polypyrrole nano-composite has been drop casted on to the SPE for the successful fabrication of one shot disposable SPE for the first time. Further, the Fe2V4O13-polypyrrole nano-composite modified SPE is used as an effective and reliable electrochemical sensor to detect As(III) present in drinking water.
2. Experimental
2.1. Synthesis of amorphous Fe2V4O13
The amorphous Fe2V4O13 was prepared according to the previously reported method [9]. Briefly, NH4VO3 (0.467 g) was dissolved in 20 ml of distill water at 60 °C. To this, Fe(NO3)3·9H2O (0.808 g) was added and stirred for 3 h. The resulting precipitate was retrieved by centrifugation and then washed with water and ethanol followed by dried at 60 °C. Finally, the resulting product was calcined at 300 °C/6 h to get amorphous Fe2V4O13.
2.2. Synthesis of Amorphous Fe2V4O13-polypyrrole Nanocomposite
The prepared amorphous Fe2V4O13 (0.10 g) was dispersed in 20 ml of distilled water under sonication. To this, 0.1 ml of pyrrole dissolved in 10 ml of water was added at 10 °C. Then, 1.0 g ammonium persulphate dissolved in 20 ml distill water was added drop wise at temperature below 10 °C. Finally, the resulting amorphous Fe2V4O13-polypyrrole nanocomposite was retrieved by centrifugation and washed with water and ethanol followed by dried at 60 °C.
2.3. Material characterization
Powder x-ray diffraction patterns (PXRD) of amorphous Fe2V4O13 and its polypyrrole composite were collected by using PANalytical X'pert PRO x-ray diffractometer. The FTIR spectra of Fe2V4O13 and its composite with polypyrrole were collected using IS5-800 Fourier transform infrared Nicolet spectrometer. The surface morphology of Fe2V4O13 and its polypyrrole composite were studied by using JEOL-5600LV scanning electron microscopy. The electrochemical studies, such as cyclic voltammetry (CV), differential pulse anodic stripping voltammetry (DPASV) have been conducted using biologic SP150 potentiostat. As(III) content present in the synthetic water solution was estimated using G.B.C. make, Avanta model, Atomic Absorption Spectrophotometer with AA Hydride System HG 3000.
2.4. Screen-printed electrode modification
The prepared amorphous Fe2V4O13-polypyrrole nanocomposite (2.0 mg) was dispersed in 2 ml of distilled water under sonication. The 10 μl of the resulting suspension was drop casted on SPE (working electrode area, 3 mm) and then dried under infra-red light. Finally, dried SPE was used for electrochemical measurements. The schematic representation of the fabrication of Fe2V4O13-polypyrrole nano-composite modified SPE is presented in scheme
Scheme 1. The schematic representation of the fabrication of Fe2V4O13-polypyrrole nano-composite modified SPE.
Download figure:
Standard image High-resolution image2.5. Analytical procedure
All experiments were carried out at room temperature, without removing oxygen from the solution. The amorphous Fe2V4O13-polypyrrole nanocomposite modified SPE was immersed in the electrochemical cell containing As(III) along with the supporting electrolyte 0.1 M HCl (scheme
Scheme 2. The schematic representation of the working of Fe2V4O13-polypyrrole nano-composite modified SPE towards the detection of As(III).
Download figure:
Standard image High-resolution image2.6. Real sample preparation
100 ppm of As(III) standard solution was prepared by using NaAsO2 in double distilled water and diluted to 1000 ml. So far, ground water containing arsenic has not been reported in southern states of India. Hence, synthetic water was prepared according to the previous literature [10, 11] where known quantities of As(III) standard solution was added to the drinking water, supplied by Bangalore water supply and sewerage board, Bangalore, Karnataka, India. Finally, As(III) content was estimated, using atomic absorption spectrophotometer, based on calibrated hydride generation method in accordance with the protocol suggested [12].
3. Result and discussion
3.1. Structural and morphological characterization
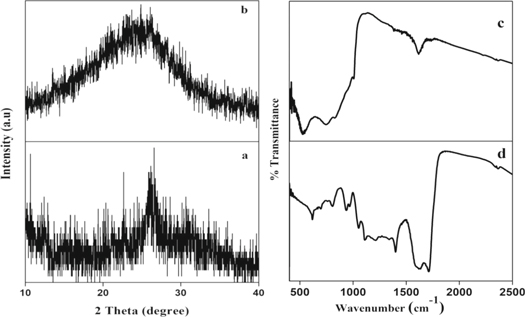
The powder XRD patterns of the prepared Fe2V4O13 and its composite were shown in figures 1(a) and (b) respectively, where the presence of broad peak between 20 and 30° confirms the amorphous nature of both Fe2V4O13 and its composite with polypyrrole. The FTIR spectrum of the amorphous Fe2V4O13 (figure 1(c)) reveals the presence of characteristic bands at 523, and 850 cm−1, wherein the band appeared at 523 cm−1 is assigned to V-O-V deformation and 850 cm−1 is assigned to bridging V-O-Fe mode. The FTIR spectrum of the composite shown in figure 1(d) exhibits a series of characteristic bands corresponding to the Fe2V4O13 and polypyrrole, indicating the formation of the Fe2V4O13-polypyrrole nano-composite. The peaks at 960 cm−1, 784, and 682 cm−1 are respectively accredited to the in-plane C=C bending and out-of-plane C-H bending in polypyrrole. The peaks appeared at 1094 cm−1 and 1040 cm−1 assigned to N+H2 in-plane vibration and combination of C-H in-plane ring bending and C=C–N deformation of the five-membered ring respectively. The peaks observed at 1550 and 1478 cm−1 are due to the pyrrole ring vibration [13–15]. Additionally, the bands 523 and 760 and 850 cm−1 appeared for Fe2V4O13 were shifted towards higher wavenumber in the composite, indicating the significant interaction between polypyrrole and Fe2V4O13.
Figure 1. PXRD pattern of (a) Fe2V4O13, (b) Fe2V4O13-polypyrrole composite, (c) FTIR spectrum of Fe2V4O13 and (d) FTIR spectrum of Fe2V4O13-polypyrrole composite.
Download figure:
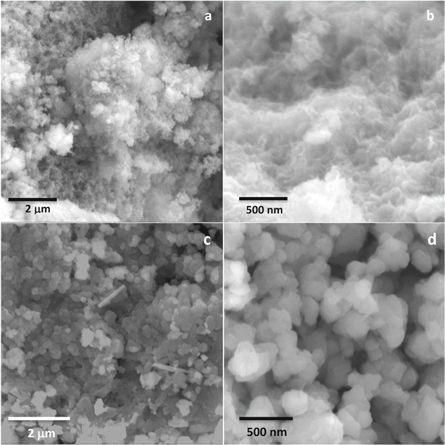
Standard image High-resolution imageThe formation of Fe2V4O13-polypyrrole nanocomposite is further confirmed by the SEM analysis. The morphology of the amorphous Fe2V4O13 and its composite with the polypyrrole, is presented in the figure 2. As observed from the figures 2(a) and (b), the amorphous Fe2V4O13 is made up of fine particles with porous structure, while the Fe2V4O13-nanocomposite, shown in the figures 2(c) and (d), exhibits the different morphology confirming the formation of composite where it is made of mono-dispersed nanoparticles with diameter of about 100–200 nm, indicating the formation of nanocomposite.
Figure 2. The SEM image of (a) and (b) Fe2V4O13 and (c) and (d) Fe2V4O13-polypyrrole nanocomposite.
Download figure:
Standard image High-resolution image3.2. Sensing of As(III) nanocomposite modified SPE
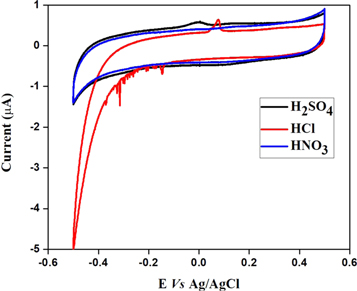
As shown in the figure 3, the CV response of the nanocomposite modified SPE towards As(III) detection exhibits well resolved sharp peak at 0.06 V in HCl and broad peak at around 0.0 V in H2SO4 electrolyte. The observed sharp/broad peak is due to the oxidation of As(0) → As(III) [16]. Whereas, no oxidation peak was observed in HNO3 electrolyte, indicating the prepared nanocomposite is sensitive towards As(III) only in HCl and H2SO4 electrolyte. Further, the nanocomposite modified SPE shows higher peak current in HCl compared to the H2SO4 and therefore further quantification studies were conducted in HCl electrolyte. The high peak current in HCl is attributed to the complexation of Cl- ions with As(III) [17].
Figure 3. Cyclic voltammogram of the Fe2V4O13-polypyrrole nanocomposite modified SPE response towards As(III) in presence of HCl, H2SO4 and HNO3.
Download figure:
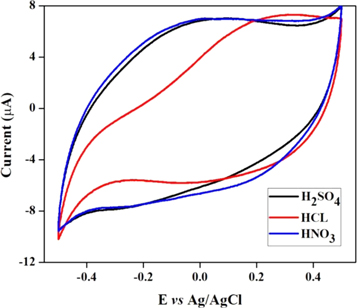
Standard image High-resolution imageThe similar CV experiments were conducted using polypyrrole modified SPE and Fe2V4O13 modified SPE separately to understand the individual effect on As(III) detection and the observed results were presented in the figure 4, where no obvious peak corresponds to the oxidation of As(0) → As(III) was observed for polypyrrole modified SPE in HCl, H2SO4 and HNO3 electrolytes. Further, no data obtained with Fe2V4O13 modified SPE since Fe2V4O13 is soluble in the acid electrolytes.
Figure 4. Cyclic voltammogram of polypyrrole modified SPE towards As(III) in presence of HCl (red), H2SO4 (black) and HNO3 electrolyte.
Download figure:
Standard image High-resolution imageThus, the presence of polypyrrole in the composite enhances the stability of Fe2V4O13 and thereby resists the solubility in acid electrolyte. Therefore, the observed response for As(III) is attributed due to the cooperative effect of polypyrrole and Fe2V4O13 where Fe2V4O13 provides electroactive surface and polypyrrole enables the required conducting path for the fast electron transport. Hereafter, the differential pulse anodic stripping voltammetry (DPASV) was used to quantify the As(III) in order to get better analytical response.
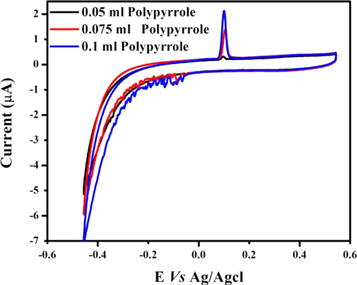
The optimized ratio of Fe2V4O13 and polypyrrole was selected based on the CV result obtained with the Fe2V4O13-polypyrrole nanocomposite prepared using different amount of pyrrole. Figure 5 shows the CV results obtained with the Fe2V4O13-polypyrrole nanocomposite prepared using 0.7 mmol, 1.0 mmol and 1.44 mmol pyrrole where we observed that the Fe2V4O13-polypyrrole nanocomposite prepared using 1.44 mmol pyrrole gives highest current density for As(III) in HCl electrolyte. Therefore, the Fe2V4O13-polypyrrole nanocomposite prepared using 1.44 mmol pyrrole was selected for further work.
Figure 5. The CV results of the Fe2V4O13-polypyrrole nanocomposite prepared using 0.7 (black line), 1.0 (red line) and 1.44 mmol (blue line) pyrrole.
Download figure:
Standard image High-resolution image3.3. Quantitative analysis of As(III) by differential pulse anodic stripping voltammetry (DPASV)
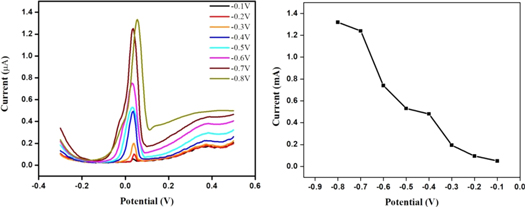
The applied potential significantly affects the current and thus the effect of applied potential on the Fe2V4O13- polypyrrole modified SPE towards the reduction of As(III) to As(0) has been carried out. The deposition potential is varied from the potential value of −0.3 to 0.8 V to evaluate the optimized value. As the potential increases from −0.1 to −0.4 V, the peak current increases without change in the shape of the peak (figure 6). However, further increase in potential also increases the peak current with change in the shape of the peak. Therefore, −0.4 V is considered as optimum value for the detection of As(III).
Figure 6. DPASV response of Fe2V4O13-polypyrrole nanocomposite modified SPE against deposition potential.
Download figure:
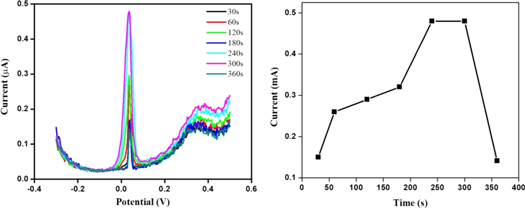
Standard image High-resolution imageSimilar to the deposition potential, the deposition time also plays an important role in the accumulation of As(III) from the bulk of the electrolyte onto the modified electrode (figure 7). Therefore, the deposition time has been varied from 30 s to 360 s where the peak current increases from 30 s to 300 s and thereafter decreases. Thus, the deposition time of 300 s has been considered as optimized value.
Figure 7. DPASV response of Fe2V4O13-polypyrrole nanocomposite modified SPE against time.
Download figure:
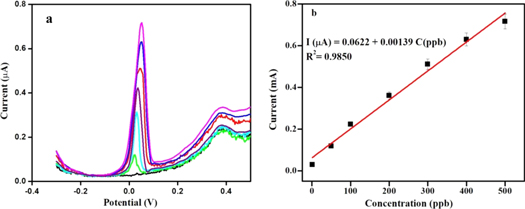
Standard image High-resolution imageThen, the DPASV response of the proposed sensor at different concentrations of As(III) was carried out under optimized conditions of deposition potential (−0.4 V) and time (300 s), where the stripping peak current increases linearly with increase in As(III) concentration up to 500 ppb without change in the peak position (figure 8(a)). The correlation coefficient for the linear fit is found to be 0.985 (figure 8(b)), indicating reasonably a good fitting.
Figure 8. DPASV response of Fe2V4O13-polypyrrole nanocomposite modified SPE against (a) concentration and (b) calibration graph.
Download figure:
Standard image High-resolution imageThe limit of detection (3σ method) is found to be 0.3 ppb, which is well below the value of 10 ppb prescribed by WHO for As(III) [18]. Further, the observed LOD and the linear range have been compared with the electrochemical methods, reported for the detection of As(III), shown in table 1 [8, 19–22], where the lower LOD and wide linear range makes the proposed sensor very attractive.
Table 1. Comparison of Fe2V4O13-polypyrrole nanocomposite response towards As(III) with reported methods.
| Sl. No | Modifier | Electrode | Linear range (ppb) | Limit of detection (3σ method) (ppb) | Reference |
|---|---|---|---|---|---|
| 1 | Gold nanoparticles | Glassy carbon | — | 0.0096 | [19] |
| 2 | Gold nanoparticle modified carbon nanotubes | Glassy carbon | 10–70 | 0.1 | [20] |
| 3 | Mercaptoethylamine | Gold | 0.2–300 | 0.02 | [21] |
| 4 | Graphene oxide-Au nanoparticles | Glassy carbon | 0.0001–0.005 | 0.20 | [22] |
| 5 | Fe3O4 microspheres-Ionic liquid composite | Screen printed carbon | 1–10 | 0.0008 | [8] |
| 6 | Fe2V4O13-polypyrrole nanocomposite | Screen printed Electrode | 0–500 | 0.3 | Present study |
NR-Not reported
The reproducibility, repeatability and stability of the nanocomposite modified SPE are also important for practical applications. The reproducibility of the proposed electrode was examined by using the same electrode for 10 repetitive measurements in 1 M HCl electrolyte containing 50 ppb of As (III) under identical and optimized conditions. The relative standard deviation was found to be ±4.4%, indicating that the analytical performance of the fabricated electrode is highly reproducible. Further, the stripping voltammograms utilizing one single electrode were measured over a period of 30 days and the maximum deviation obtained was 4.5%, indicating the excellent stability and repeatability of the proposed electrode. Owing to this high selectivity and sensitivity with low detection limit and wide linear range the proposed sensor could be used for real sample analysis.
In order to evaluate the proposed analytical method, the Fe2V4O13-polypyrrole nanocomposite sensor has been successfully applied for the detection of As(III) present in synthetic water. The results obtained by the proposed method are in good agreement with results obtained by atomic absorption spectrophotometry (AAS) data shown in table 2. Thus, the observed result shown in the table 2 reveals that the proposed sensor could be used as a potential sensor for As(III) detection.
Table 2. Application study: Determination of arsenic (III) from different environmental samples.
| Found (ppb) | Recovery (%) | |||||
|---|---|---|---|---|---|---|
| Sample | Originally found (ppb) | Added (ppb) | Proposed method | AAS method | Proposed method | AAS method |
| Synthetic water sample | 50 | 20 | 69.55 | 68.95 | 99.35 | 98.50 |
4. Conclusions
In summary, high performance amorphous Fe2V4O13-polypyrrole nanocomposite was prepared by simple wet chemical method. The prepared Fe2V4O13-polypyrrole nanocomposite has been used as electrode modifier and thus SPE was modified. The fabricated nanocomposite nanocomposite modified SPE was used to detect As(III) at ultra-trace level where several parameters were optimized to get better sensing results. The proposed SPE exhibits low detection limit of 0.3 ppb.
Acknowledgments
The author, Ashoka S (SA) acknowledges the Science and Engineering Research Board (ECR/2017/000743), Government of India, for financial support. The authors also express sincere thanks to the Vision Group of Science and Technology (GRD-743/2017-18), Government of Karnataka.