Abstract
The vermiculite (VMT), as a natural hydrate mineral, is one of the most competitive candidates of two dimensional (2D) mateirals in various areas including waste water treatment, energy-saving building, green agriculture, etc. Notably, 2D expanded VMT offers a nano-sized multilayered structure, which has triggered far-ranging interest in the development of 2D nanomaterials and processing capabilities to fulfill the requirements of environmental adsorption materials, thermal storage materials, high efficient catalysts and so on. VMT as an additive material is commonly used to enstrong mechanical, thermal and flame retardant properties, particularly for polymer materials. Additionally, some emerging materials derived from VMT, such as 2D silica nanomesh, 2D VMT-based layered double hydroxide (LDH), lithium silicates, silicon carbide ceramic material, have been well engineered to expand its application ranges. In this review, we focus on VMT-based nanomaterials, summarize physical and chemical behaviors, and discuss mechanism and problems encountered in its application. The major goal is to highlight the recent progress of 2D VMT in various practical and potential applications.
Export citation and abstract BibTeX RIS
Abbreviations
| 2D | Two dimensional |
| 3MPS-VT | 3-Mercaptopropyl Trimethoxysilane |
| AR | AstrazonRed |
| BET | Brunauer-Emmett-Teller |
| BR9 | Basic Red 9 |
| CA-PA-SA | Perovskite-type Stearic acid |
| CHT | Chitosan |
| CIP | Ciprofloxacin |
| CTA | Cysteamine Hydrochloride |
| CV | Crystal Violet |
| CYS | L-cysteine |
| De-NOx | Denitrification |
| DSC | Differential Scanning Calorimetry |
| EDX | Energy Dispersive X-ray |
| EML-VMT/EVM | Expanded Multilayered Vermiculite |
| HTT | Hydrothermal Treatment Process |
| LA | Lauric Acid |
| LA-PA-SA | Lauric Acid-Palmitic Acid-Stearic Acid |
| LDH | Layered Double Hydroxide |
| LDO | Layered Double Oxide |
| MB | Methylene Blue |
| MHCs | Monolithic Honeycomb Catalysts |
| MIC | Minimum Inhibitory Concentration |
| OTC | Oxytetracycline |
| OVMT | Organically Modified Vermiculite |
| PEG | Polyethylene Glycol |
| PFOS | Perfluorooctane Sulfonate |
| PLLA | L-Lactic Acid |
| PMMA | Polymethylmethacrylate |
| PyY | Pyrrolidine Y |
| SAPs | Superabsorbent Polymers |
| SCR | Selective Catalytic Reduction |
| SEM | Scanning Electron Microscope |
| SiNSs | silica nanosheets |
| TES | Thermal Energy Storage |
| TOF | Turnover Frequency |
| TSC | Transpired Solar Collector |
| VMT/VT/VER/Verm | Vermiculite |
| VMT-SiO2 | Vermiculite-Based SiO2 |
| XRD | X-Ray Diffraction |
1. Introduction
Vermiculite (VMT) is a kind of natural hydrate clay mineral, which is mainly located in South Africa [1], Unite State [2, 3], Brazil [4] and China [5]. VMT processes two-dimensional (2D) layered structure that consists of lamella (silicon oxygen tetrahedron, magnesium oxygen octahedron, etc) and interlaminar cation (hydroxyl molecules) [6]. Generally, VMT is easy to expand more than 20 times size by heat treatment [7], microwave irradiation [8], hydrogen peroxide expansion [9], etc. Moreover, surfactants have been widely employed to exfoliate VMT into the stratified structure of VMT [10, 11].
Nowadays, the VMT is one of the most competitive candidates of two dimensional (2D) mateirals in various areas including waste water treatment, energy-saving building, green agriculture, high efficiency catalysis, etc [12]. Interestingly, 2D expanded VMT offers a nano-sized multilayered structure, which has been extensively explored the development of 2D nanomaterials and processing capabilities to meet environmental adsorption materials [13, 14], mineral−water interaction materials [15], high performance catalysts [16], gamma radiation shielding material [17], machinable glass-ceramics [18], feed additive [19, 20], furthermore, VMT as an additive material is widely used to enhance mechanical [21], thermal [22], flame retardant, light weight composite [23], noise abatement [24], non-biological [25], scale inhibitor [26] and oxygen barrier properties [27], particularly for polymer materials applications. In addition, some emerging materials derived from VMT, such as 2D silica nanomesh [28], 2D VMT-based layered double hydroxide (LDH) [29], lithium silicates [30] and silicon carbide ceramic material [31], etc.
This review focuses on VMT-based nanomaterials, such as expanded multilayered VMT, environmental adsorption materials, VMT-supported catalysts, bacteria carriers and antibacterial materials, VMT-modified inorganic nanocomposites, VMT-modified polymer nanocomposites, mechanical nanocomposites and VMT-derived nanomaterials. Moreover, we summarize physical and chemical behaviors, and discuss mechanism and problems encountered in its application. The primary aim is to highlight the recent progress of 2D VMT-based nanomaterials in various practical and potential applications.
2. Expanded multilayered VMT
Vermiculite is able to expand more than 20 times size after abrupt heating at the elevated temperature, and causes a considerable pressure in the expand process. Expanded multilayered VMT possesses versatile properties such as adsorptivity, high cation exchange capacity and chemical and mechanical stability, etc. In this section, we mainly introduce the applications of expanded multilayered VMT in excavation and materials preparation fields.
2.1. Rock cracking materials
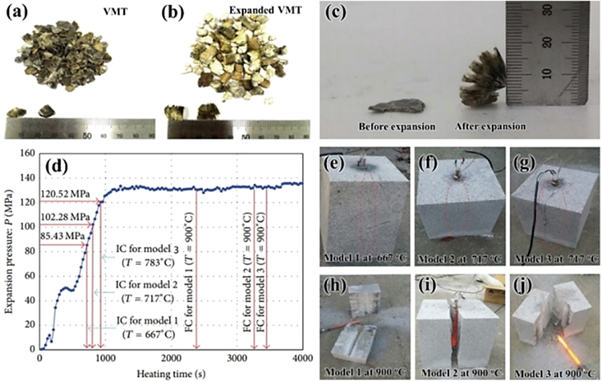
Rock cracking material is a powdery substance composed of inorganic compound or organic compound, which is cracked by a specific chemical reaction or by heating to generate the high expansion force. As shown in figures 1(a)–(c), VMT are rapidly expanded in volume when they receive thermal energy. In the expanding process, the expansion pressure is large enough to break hard and massive granite rock. Ahh et al [32, 33] found that this novel vibration-free excavation method utilizing the expansion VMT showed a potential application in construction site. Compared with traditional explosion and shield excavation methods, VMT expansion excavation method can overcome the disadvantages including vibration, noise, extened schedule, and high costs. this new method could be used to explore underground spaces and satisfy the demand of sharply increasing city residents. Figure 1(d) presented internal expansion pressure occurring at the inside of the perforated cylinder hole during heating. After 720 s, the model 1 cracked at 667 °C and sharp cracked at 85.43 MPa internal expansion pressure. Then model 2 and model 3 cracked initiation process at 717 °C and 783 °C, respectively (figures 1(f), (g)), with the expansion pressure increasing to 102.28 MPa and 120.52 MPa, respectively. The internal expansion pressures can be accurately estimated before the value of 130 MPa. As shown in figures 7(h)–(j), the ultimate failure occured along the critical path which coincided with the propagation of the initial crack.
Figure 1. (a)–(c) Thermal expansion of the VMT. (d) Internal pressure of the perforated cylinder holes. (e)–(g) Initial cracking (IC) images of massive granite subjected to uniform internal pressure. (h)–(j) Final cracking (FC) images of massive granite subjected to uniform pressure [32]. (Open access).
Download figure:
Standard image High-resolution image2.2. 2D space-confined layed materials
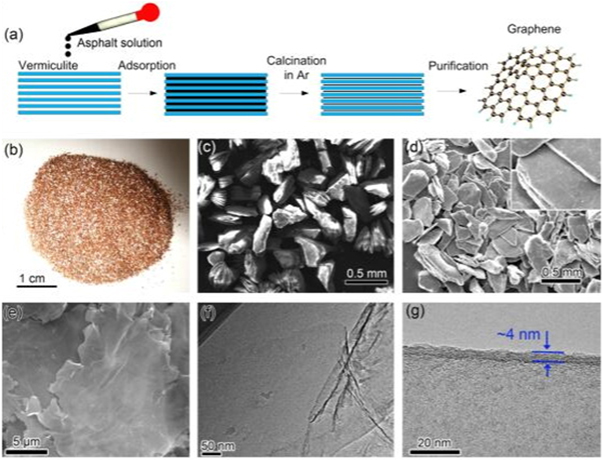
The figure 2(a) shows that expanded multilayered vermiculite (EML-VMT) can adsorb asphaltene molecules onto its surfaces or into its interstices. Xu et al [34] discussed that the use of expanded multilayered vermiculite as a hard template to design the enhanced electrochemical performance graphene from asphaltene molecules. The EML-VMT shows red color due to the Fe-containing VMT surface (figure 2(b)) and presents a russet powder a layered structure in width of 200–500 μm (figure 2(c)). The layered structure was retained after the asphalt adsorption and carbonization (figure 2(d)). After the removal of vermiculite substrates, the graphene sheets exhibit a flat laminar morphology in width of tens of microns ((figures 2(b) and (f)). The orderly graphene layers can be clearly observed and the thickness of the graphene sheets is ∼4 nm, corresponding to 8–10 graphene layers (figure 2(g)).
Figure 2. (a) Illustration of the synthesis process of graphene sheets from asphalt adsorbed by vermiculite. (b) A photo of the vermiculite. (c) The SEM image of the vermiculite. (d) The SEM image of the vermiculite after asphalt adsorption and carbonization. The inset of (d) shows a high resolution SEM image of the material. (e) The SEM of the as-prepared graphene sheets. (f),(g) The TEM images of the as-prepared graphene sheets (after purification) [34] (© 2013, with permission from Elsevier).
Download figure:
Standard image High-resolution imageNowadays, lithium-sulfur batteries represent one of the most efficient battery systems given that their high theoretical energy density. Unfortunately, the further development of lithium-sulfur batteries is largely constrained by the lithium dendrite and shuttle effect inducing parasitic reactions. Xu et al [35] fabricated the 2D vermiculite nanosheets as the separator surface coating of lithium-sulfur batteriesto alleviate shuttle effects through electrostatic repulsion and steric hindrance. Interestingly, in 2019, Wu et al [36] firstly reported that natural vermiculite was used as the host material for the sulfur-containing positive electrode of lithium-sulfur battery to reduce the dissolution of polysulfides and inhibit the shuttle effect via the cations on the surface of the vermiculite act on polysulfide anions.
2.3. Porous VMT
Vermiculite is typical layered aluminosilicate clay, which was mainly used as acoustic and heat insulation filler for buildings in the early stage. With the challenge of promoting practical applications of VMT, the structure of VMT should be well designed to construct porous architectures with sufficient void spaces and high specific surface area. As such, Chemical modification (i.e. acid etching [37], intercalation, pillaring) is a facile, effective, and widely employed means for improving the structures and properties of 2D VMT to fulfill the requirements of modern industry. VMT decorated with aluminum and lanthanum can obtain the mesoporous structure for adsorption and catalytic processes. The crystallinity of the synthesized material was characterized by XRD. The effectiveness of the method used was confirmed, and the morphology was subjected to nitrogen adsorption analysis (BET), as presented in table 1. The results confirmed the porosity of the composite [38].
Table 1. Macroscopic properties of vermiculite and modified vermiculite.
| Mateirals | specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | References |
|---|---|---|---|---|
| Raw Vermiculite | 6 | 0.02 | — | [37] |
| H-Vermiculite | 198 | 0.22 | — | [37] |
| VNAT | 7.1 | — | 3.48 | [38] |
| Al13PILV | 230.1 | — | 2.19 | [38] |
| LaAL12PILV | 190.9 | — | 4.57 | [38] |
| La2Al11PILV | 91.1 | 5.38 | [38] |
3. Environmental adsorption materials
Developed microstructure, thermochemical stability and low cost of expanded multilayered VMT opens an avenue in numerous potential applications for environmental adsorption materials. Furthermore, with structure and properties rationally fabricated, the VMT not only retain the intrinsic properties but also enhance adsorption capacity as well as selective adsorption and rapid adsorption. this part aims to summarize the preparation and utilization of VMT-based environmental adsorption materials in wastewater treatment system, including heavy metal ion-containing wastewater, fluoride wastewater, dye wastewater, ammonia-nitrogen and phosphorus wastewater, pharmaceutical wastewaters, as presented in table 2.
Table 2. Adsorption properties of environmental adsorption materials.
| Adsorption materials | Waster water | Adsorption capacity or seletivity | References |
|---|---|---|---|
| VMT | Cr(VI) | adsorption coefficient Kd=0.74 × 105μgkg−1 | [39] |
| MgAL LDH/VMT | Cr(VI) | >70% Cr(VI) removal percentage @10th recycle | [40] |
| VMT-H2O2 | Cd(II) | 100% removal percentage after 168 h | |
| Pb(II) | ∼90% removal percentage after 168 h | [41] | |
| BAL-VMT | Hg(II) | 8.57 mg g−1 @ pH = 4,5 | [42] |
| 3-MPS-VMT | Hg(II) | 21 ± 2μmol/g@pH = 6.0 ± 0.1, 25.0 ± 0.5 °C | [43] |
| O-VMT65%CTS | Cd(II) | 141 mg g−1 @ 30 °C, pH = 7, 300 mg l−1 | [44] |
| VMT | Cd(II) | 79.9 mg g−1 @ 35 °C, pH = 7, 300 mg l−1 | [45] |
| VMT | Ce(III), Ce(VI) | — | [46] |
| CTS-VMT | As(III) | 72.2 mg g−1 @ 20 °C, pH = 5 | [47] |
| Fe3O4/VMT | Pb(II) | 70.4 mg g−1 @ 25 ± 1 °C, pH = 4,5, 300 mg l−1 | [48] |
| VMT | Pb(II), Cd(II), Cu(II), Ni(II) | 0.238, 0.099, 0.199 and 0.115 mmol g−1 | [49] |
| VMT | Cr(III), Zn(II), Mn(II) | Cr(III)>Zn(II)>Mn(II) | [50] |
| VMT | Zn(II), Cu(II) | Zn(II)>Cu(II) | [51] |
| FeOx/VMT | Ni(II), Cu(II) | 101.3, 59.7 mg g−1 | [52] |
| HDTMA-VMT0.2 M | F- | 2.36 mg g−1 | [58] |
| VMT-Fe20 | F- | 14.43 mg g−1 | [59] |
| VMT-Nitric-Citric (VNC) | Astrazon red, methylene blue | 100.8 ± 0.8, 150 ± 4 mg g−1 | [60] |
| VMT-H-OH (VN-OH) | Astrazon red, methylene blue | 127 ± 2, 203 ± 4 | [61] |
| VMT-H-OH (VN-OH) | Astrazon red, methylene blue | 67 ± 3, 115 ± 2 mg g−1 | [62] |
| P-VMT | Pyronin B (PYB) | 33.0 mg g−1 | [63] |
| Ethylenediamine- VMT | RN | 11.02 mg g−1 | [64] |
| CTS-PAA-10%VMT | methylene blue | 1612.32 mg g−1 | [65] |
| VMT | methylene blue | 0.076 mol g−1 @ 25 °C | [67] |
| Expanded vermiculite | BR9 | 7.657 × 10-5 mol g−1 @ 25 °C | [68] |
| VMT | PyP | 2.145 mg g−1 | [69] |
| VMT | NH4+ | 1.79 mg g−1 in the presence of sulfate | [70] |
| VMT-packed electrochemical | NH4+ | 30 mgN L−1 ammonia removal rate was 89% | [71] |
| MEV | phosphate | 714.3 mg kg−1 | [73] |
| chitosan-g-poly(acrylic acid)/vermiculite | phosphate ions | 22.64 mg g−1 | [74] |
| PC-VER | OTC, CIP | 24.03, 23.48 mg g−1 | [78] |
| Fe3O4/F-VT | PFOS | 1127 mg g−1 | [79] |
3.1. Heavy metal ion-containing wastewater
Heavy metal wastewater seriously damages water, plants and animals in water, and thus may affect human health and even life through the food chain. Therefore, researches commit oneself to seek for environmentally friendly technologies to reduce the pollution of sediments in water. In particular, environmental adsorption materials consisiting of natural clay are fabricated as low-cost and eco-friendly materials to eliminate heavy metals ion in aqueous solutions.
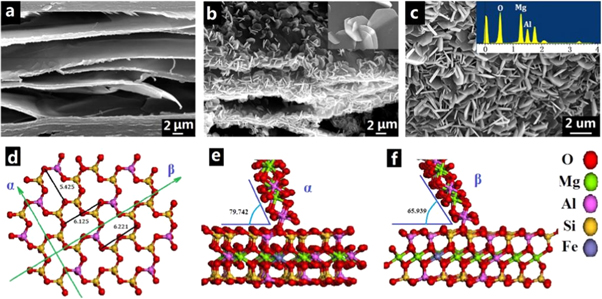
In terms of the adsorption of Cr in wastewater, significant research have focused on advanced and well designed vermiculite based sorbents from expanded vermiculite to vermiculite based composites. Marcos et al [39] found that thermo-exfoliated vermiculite is very suitable for recycling trace amounts of Cr(III) in high salt content water with high adsorption coefficient (K-D = 0.74 × 105 μg kg−1). As SEM images shown in figure 3(a), VMT exhibits flat and smooth surface, which is beneficial to fabricate three dimensional (3D) structural material. Tian et al [40] reported the growth of 3D MgAl-LDH (layered double hydroxide) (figures 3(b) and (c))with two direction lattice match ways (figures 3(e) and (f)) on the surface of VMT via the in situ epitaxial growing technique. The as-prepared 3D MgAl-LDH/VMT composite delivered better Cr (VI) adsorption performance and still exhibited a removal rate of over 70% after 10 cycles. The above studies reveal that both expanded vermiculite and vermiculite-base composites can potentially be used in the treatment of Cr (III) and Cr (VI) wastewater.
Figure 3. (a) SEM images for edge of the VMT. (b) MgAl–LDH/VMT sample, and (c) the surface of VMT sample (Inset: EDX of the MgAl–LDH material on the VMT) (Inset: EDX of the MgAl–LDH on the VMT). (d) the surface of Si–O tetrahedral of VMT, (e) α growth mechanism, and (f) β growth mechanism [40]. (© 2016, with permission from Elsevier).
Download figure:
Standard image High-resolution imageThe modification of vermiculite is one of the most frequently used techniques for obtaining functional materials with high void structure as well as high selectivity and fast adsorption. Typical modification methods, such as acid modification, peroxide modification, organic modification, have been extensively employed to synthesize VMT-based sorbents. For example, Hashem et al [41] treated VMT with 0.5 mol HCl and 30% H2O2 solution respectively, activated vermiculite expansion factors were 2.5 and 14.83, respectively. which exhibited the removal efficiency of VMT-H2O2 for cadmium and lead ions after 168 h is 100% and 90% higher than 66.7% and 47% of VMT-HCl, respectively. Tran et al [42] reported that BAL-VMT was prepared by grafting a thiol and a hydroxyl binfunctional group dimercaptopropanol (BAL) onto the vermiculite, the pH value and other cations of the solution have a great influence on the adsorption of Hg(II). In addition, Nascimento et al [43] studied Na+ exchange vermiculite (Na-VMT) was modified with L-cysteine (CYS), cysteamine hydrochloride (CTA) and 3-mercaptopropyltrimethoxysilane (3-MPS), respectively. The column adsorption capacity is 3MPS-VT > CTA-VT > CYS-VT > Na-VT, and 3-MPS-VMT has high selectivity and high adsorption capacity to Hg (II).
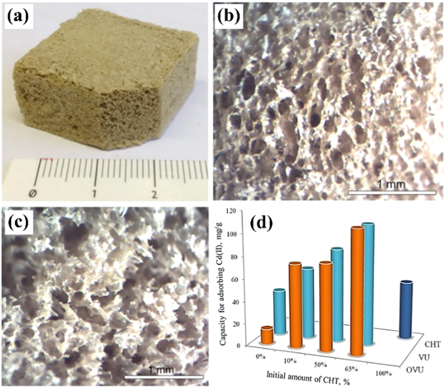
Chitosan had also been used as an adsorbent to uptake heavy metal ion, but its instability in solution restricts further application prospects. Fortunately, the combination of chitosan and clay VMT not only heightens the resistance of chitosan in solution but also significantly improves adsorption capacity. Chitosan and VMT have reported two different ways of connection. For example, Macroporous bionanocomposite foam (figure 4(a)) was fabricated by the intercalation of chitosan into interlayer of organically modified vermiculite to remove Cd (II), and better visualized in those images obtained with an optical microscope (figures 4(b), (c)) figure 4(d) exhibits that the chitosan vermiculite biocomposite with the highest chitosan content has a higher adsorption capacity value. The experiment showed that the adsorption capacity was increased by raising the solution pH, which most likely favors a chelation mechanism to absorb Cd (II) [44]. The adsorption mechanism of Cd (II) on vermiculite is mainly through ion exchange and electrostatic attraction [45]. Klika et al [46] mainly focused on the adsorption mechanism of Ce (III) and Ce (IV) on vermiculite. The composite of chitosan and VMT was assembled through ionic crosslinking of oxygen atoms of surface of vermiculite decorated with oxalic and the amide groups of chitosan [47], which exhibited maximum adsorption capacity of As (III) was 72.2 mg g−1. However, the adsorption/desorption performance was decreased by 40% after 10th cycles. To enhance the cycling stability of VMT-based environmental adsorption materials. Yao et al [48] adopted the solvothermal method to produce Fe3O4/VMT with superparamagnetic (endowing the ability of magnetic separation) and large surface area, measuring the maximum adsorption capacity of Pb (II) to reach 70.4 mg g−1, which was almost 2 times higher than that of VMT.
Figure 4. [Rename CTS to CHT] (a) Digital image of chitosan–vermiculite macroporous foam. Optical microscope images of the surface of (b) VU 65% CHT and (c) OVU 65% CHT bionanocomposites. (d) Comparison of the capacities of OVU, VU, CHT and bionanocomposite foams for adsorbing Cd (II) from aqueous solution [44]. (© 2015, with permission from Elsevier).
Download figure:
Standard image High-resolution imageThe adsorption of VMT on various metal ions in solution is competitive, the adsorption selectivity of VMT thus has attracted extensive attention. The competitive adsorption of vermiculite to the mixed system of Pb(II), Cd(II), Cu(II) and Ni(II) suggests that the total metal adsorption is distributed among the four elements [49]. Inglezakis et al [50] measured the Mn2+, Zn2+ and Cr3+ adsorptions in solution by vermiculite, and the selectivity of vermiculite obtained from batch and fixed bed experiments was Cr3+ > Zn2+ > Mn2+. In 2017, De Freitas et al [51] reported expanded VMT has high selectivity to zinc in Zn2+ and Cu2+ two-component solutions. Nashtifan et al [52] prepared FeOx/VMT composites for the removal of Ni2+ and Cu2+ in solution. The extended Langmuir model accorded well with the competitive adsorption of Ni2+ and Cu2+, and The maximum adsorption capacities of FeOx/VMT for binary systems Ni2+ and Cu2+ were measured to be 101.3 mg g−1 and 59.7 mg g−1, respectively. Jimenez et al [53] reported a kinetic model of the adsorption of metals by natural mineral vermiculite and found that the adsorption was rapid during the first 10 min of exposure (more than 50% of equilibrium adsorption had occurred), and the rate thereafter was much slower. The Elovich model is most suitable for Ni2+ and Pb2+ with a determination coefficient R2 higher than 0.9.
3.2. Cation exchange capacity of Cesium
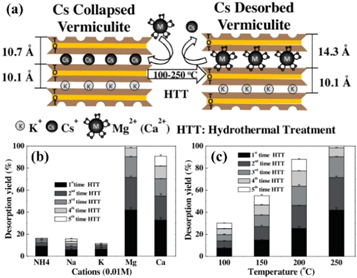
Vermiculite has a strong ability to fix cesium, but it is radioactive, what makes it imperative to remove the cesium. Grinding as a pretreatment step for acid-activated vermiculite to reduce the environmental impact typically is used to enhance mineral adsorption capacity [54]. As shown in figure 5(a), desorption of cesium from collapsed interlayer regions in vermiculite by hydrothermal treatment with divalent cations. Yin et al [55] studied how Cs+ desorption is affected by various substitution cations at different treatment temperatures, it is concluded that the diffusion of various replacement cations in the interlayer space at different hydrothermal treatment temperatures enhances desorption of Cs, leading to subsequent delamination and easily release of Cs+ (figures 5(b), (c)). Due to the large amount of cations and environmentally friendly characteristics of seawater, the introduction of seawater into the hydrothermal treatment process (HTT) can promote desorption of cerium ions in vermiculite by the cation exchange mechanism of ions under conditions [56]. Motokawa et al [57] explained the effect of cerium adsorption on the mesoscopic structure of clay mineral vermiculite and weathered biotite in suspension. The authors' analysis shows that the number of stacked layers of vermiculite is reduced by Cs+ addition, while WB is increased. In addition, the average distance between lamellar spacing of vermiculite in the suspension is greater than weathered biotite, which reflects the different conditions of the Cs+ intercalation. These findings provide basic insights that are important for predicting cesium in contaminated areas and extracting cesium from soil.
Figure 5. (a) Desorption of cesium from collapsed interlayer regions in vermiculite by hydrothermal treatment with divalent cations, (b) Sequential Cs desorption from Cs saturated Verm as functions of various cation species by hydrothermal treatment. (c) Sequential Cs desorption from Cs saturated Verm as functions of temperature by hydrothermal treatment with Mg2+ [55]. (© 2016, with permission from Elsevier).
Download figure:
Standard image High-resolution image3.3. Fluoride wastewater
Groundwater is the main source of water, and excessive fluoride in groundwater is prone to cause fluorosis, which has been a concern for decades. The limit by the World Health Organization is that the fluoride content in groundwater is reduced to 1.5 mg l−1. Ologundudu et al [58] studied the effect of cationic surfactant cetyltrimethylammonium bromide on the defluorination performance of clay mineral vermiculite. It was found that modified vermiculite can effectively reduce the tolerance of fluoride in groundwater. Compared with the Langmuir adsorption isotherm, the adsorption is more in line with the Freundlich model and the adsorption capacity is 2.36 mg g−1. Wei et al [59] prepared three kinds of iron oxide modified vermiculite (Verm-Fex, x = 5,10,20) by coprecipitation method. It was found that the maximum adsorption capacity (qmax) of Verm and Verm-Fex (x = 5, 10, 20) to fluorine was 3.18, 6.76, 9.27 and 12.43 mg g−1 at pH = 5.0, respectively. It can be seen that Verm-Fex (especially products with higher iron content) has higher adsorption performance for fluorine in the environment than Verm.
3.4. Dye wastewater
At present, the total annual production of dyes in the world is more than 1.5 million tons, of which more than 50% is used for textile dyeing; 10% to 20% of dyes are discharged as waste. The color of printing and dyeing wastewater is particularly serious, and it is difficult to remove it by general biochemical methods. Colored water also affects the transmission of sunlight, which is not conducive to the growth of aquatic organisms. When the chromaticity is removed by chemical oxidation, although the chromophore of the water-soluble dye is destroyed and discolored, the influence of the residue still exists. Most of the printing and dyeing wastewater enters the farmland, which will make the land saline. The sulfate of the dyeing wastewater can be converted into sulfide under the reducing condition of the soil to produce hydrogen sulfide. Therefore, the treatment of dye wastewater is imminent.
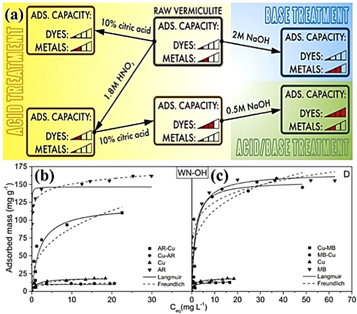
Modified vermiculite is a low cost, efficient and sustainable dye adsorbent. Stawinski et al [60] used nitric acid and citric acid modified vermiculite to adsorb astrasin red (AR) and methylene blue (MB) were 100.8 ± 0.8 mg g−1 and 150 ± 4 mg g−1, respectively, much higher than 54 ± 1 mg g−1 and 55 ± 2 mg g−1 of vermiculite. Stawinski et al [61] also found that the acid-base co-modified vermiculite has a higher adsorption capacity for dyes than acid-modified vermiculite. At the same time, Stawinski et al [62] examined the adsorption properties of acid/base treated vermiculite for single and two-component solutions of cationic dyes AR and MB and Cu2+ (figures 6(b), (c)). It was found that the raw vermiculite has low adsorption capacity for dyes and metals, but the acid/base treated vermiculite has good adsorption capacity for dyes, while the maximum adsorption capacity for Cu2+ does not change compared with the starting materials (figure 6(a)). Topark et al [63] chemically modified vermiculite with phosphoric acid to prepare P-VMT. It was found that the ability of P-VMT to remove xanthine-derived dye acetone B from aqueous solution was higher than VMT. De Queiroga [64] used ethylenediamine to modify vermiculite under different conditions, and found that vermiculite modified by ethylenediamine has good adsorption capacity for anionic dyes. Therefore, the application of modified vermiculite in dye wastewater provides a new perspective.
Figure 6. (a) Simultaneous removal of dyes and metal cations using an acid, acid-base and base modified vermiculite, (b), (c) Equilibrium experiments data for single component solutions adsorption of Cu2+, AR and MB (Cu, AR, MB, respectively) and for multi -component solution adsorption of Cu2+ (Cu-MB and Cu-AR), of AR (AR-Cu) and of MB (MB-Cu) with fitted models, for acid/base (WN-OH) treated vermiculite [62]. (©2016, with permission from Elsevier).
Download figure:
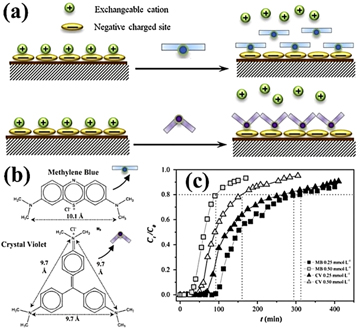
Standard image High-resolution imageVermiculite-based composites can also be used to remove dyes. Liu et al [65] used a series of chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites to remove MB from aqueous solutions. It was found that the adsorption capacity of the dye increased with the increase of pH, contact time and initial dye concentration, but the adsorption capacity decreased with the increase of temperature, ionic strength and sodium dodecyl sulfate concentration in the presence of surfactant. Shapkin et al [66] prepared polyethylene and vermiculite composites and found that chemically modified composites increased adsorption capacity. In addition, the molecular structure of dye, initial dye concentration, pH and other factors also have an effect on the adsorption process. Yu et al [67] studied the adsorption behavior of cationic dyes methylene blue (MB) and crystal violet (CV) on natural vermiculite, and explored the effect of molecular structure on the adsorption of cationic dyes (figures 7(a), (b)). The breakthrough curves of different influent concentration are shown in figure 7(c), Explaining that the molecular structure of the dye has an effect on the adsorption process. Duman et al [68] found that the adsorption amount of expanded vermiculite to Basic Red 9 (BR9) increased with the increase of initial dye concentration and pH value, but decreased with increasing temperature. Topark et al [69] studied the adsorption of pyrrolidine Y (PyY) in aqueous solution from contact time, initial dye concentration, pH, adsorbent concentration and solution temperature.
Figure 7. (a) Schematic representation of the molecular structure effect on adsorption process of MB and CV. (b) Molecular structure and size of the cationic dyes: methylene blue and crystal violet (the data of molecular size were calculated by ChemOffice software after the optimization with MP2/6–31 g(d) method). (c) Effect of influent concentration on the breakthrough curves of MB and CV [67]. (© 2015, with permission from Elsevier).
Download figure:
Standard image High-resolution image3.5. Ammonia-nitrogen and phosphorus wastewater
High contents of ammonia nitrogen and phosphorus in water bodies can lead to water pollution such as eutrophication and death of aquatic organisms. Therefore, how to prevent and manage is important. Mazloomi et al [70] studied ammonium (NH4+) of simulated wastewater in vermiculite adsorption. The kinetic analysis showed that the adsorption rate control procedure of vermiculite for NH4+ was heterogeneous chemical adsorption. Li et al [71] used a vermiculite-filled electrochemical reactor and found that both the adsorption of ammonia and the electrolysis of ammonia were present in the reactor. Under the condition of 2 A and 6 min residence time, the initial 30 mgN L−1 ammonia, the removal rate was 89% and the current efficiency was 25%. Zhang et al [72] found that the addition of vermiculite to nitrogen fertilizer can effectively maintain the NH4+-N retention time. Therefore, the addition of vermiculite is a means of controlling the eutrophication of water bodies and reducing the cost of agriculture. In the removal of phosphorus-containing wastewater, Lee et al [73] tested their ability to remove phosphate in aqueous solution using a floating adsorbent made of expanded vermiculite (EV). The maximum adsorption capacity of MEV at 380 °C was 714.3 mg K−1g−1. Zheng et al [74] prepared chitosan-g-polyacrylic acid/vermiculite composites by water-dispersion polymerization. The results showed that the maximum adsorption capacity of phosphate ions was 22.64 mg g−1, and the adsorption capacity was depended on pH values.
3.6. Pharmaceutical wastewater
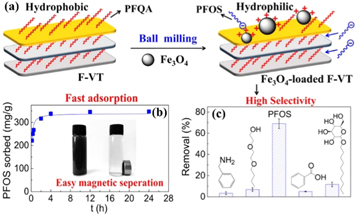
Vermiculite also has good potential for adsorption and removal of antibiotics. Tri et al [75] found four stable configurations of chloramphenicol on slab models adsorbing the surface of vermiculite. Vermiculite modified by organics is a conventional means to increase the amount of adsorption in pharmaceutical wastewater. Pouya et al [76, 77] organically modified vermiculite with cetyltrimethylammonium bromide to optimize the amount of adsorbent and initial pH and contact time and evaluate batch kinetics and equilibrium isotherms. Liu et al [78] synthesized a new type of organoclay by embedding phosphatidylcholine into vermiculite interlayer or adsorbing its surface, and PC-VER was investigated for oxytetracycline (OTC) and ciprofloxacin (CIP) adsorption characteristics. In addition, Du et al [79] synthesized a novel magnetic fluorination adsorbent of perfluorooctane sulfonate (PFOS) with high selectivity and rapid adsorption by simple ball milling of Fe3O4 and vermiculite loaded with cationic fluorinated surfactant (figure 8(a)). The supported Fe3O4 nanoparticles increased the dispersibility of fluorinated vermiculite (F-VT) and magnetic separability in aqueous solution (figure 8(b)). The used Fe3O4-loaded F-VT was successfully regenerated by methanol and reused five times without reduction in PFOS removal.
Figure 8. (a) Schematic presentaion of preparation of magnetic flu orinated vermiculite adsorbent. (b) Fast adsorption and easy magnetic separation of adsorbent from wastewater. (c) The selectivity of magnetic fluorinated vermiculite adsorbent [79]. (©2017 American Chemical Society).
Download figure:
Standard image High-resolution image4. VMT-supported catalysts
The earth-abundant and environmental benignity as well as the intriguing structural of two-dimensional clay vermiculite aslo open up its place in potential application for catalytic carrier. A variety of VMT-supported catalysts have been prepared via various well engineered approaches, correspondingly, these VMT-supported catalysts have been extensively used the significant photocatalytic and heterogeneous catalytic system. In this section, we aim to summarize the recent progress on the utilization of VMT-supported catalysts, as presented in table 3.
Table 3. VMT-supported catalysts.
| Catalysts | Methods | Reaction Types | References |
|---|---|---|---|
| VMT | — | Photocatalytic hydrogen production | [87] |
| 5%CdS/VMT | One-step method | Photocatalytic hydrogen production | [88] |
| KNbO3/VMT | In-situ hydrothermal process | Photocatalytic dye degradation | [89] |
| TiO2/VMT | Electrospinning | Photocatalytic dye degradation | [90] |
| MnOx-Fe2O3/VMT-MHC | Extrusion molding | Selective catalytic reduction of NOx by ammonia | [96] |
| Ni/VMT(MIAS) | Microwave irradation | Carbon oxide methanation | [9] |
| PIM-Ni/PVMT | Plasma irradation | Carbon oxide methanation | [104] |
| HgCl2/EML-VMT-C | Incipient wetness impregnation | Acetylene hydrochlorination | [105] |
4.1. Photocatalytic catalysts
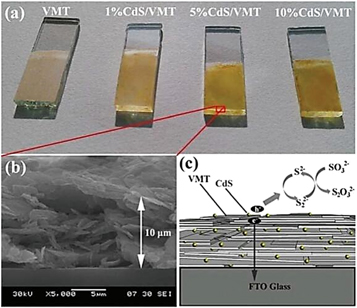
In recent years, photocatalysts for both degradation of organic pollution and hydrogen production have been widely considered as a promising way to mitigate the increasingly serious problems of environmental pollution and energy crisis. The photocalalytic efficiency is determined by the balance of thermodynamics and kinetics of charge carriers generation, charge separation and migration to the catalyst surface; and oxidation and reduction reaction on the catalyst surface [80–83]. To date, some Fe-containing materials which have high active to photocatalytic water splitting under visible light irradiation have been well designed [84–86]. Benefiting from the natural clay mineral (including affluent Fe content) and high quality nanosized structure, the VMT catalysts sensitized by organic dyes was firstly reported to actively achieve water splitting by Zhang and his coworkers [87], even the quantum efficiency of the VMT catalysts are not high. To further enhance the quantum efficiency of VMT catalysts, the combination of VMT with semiconductor photocatalysts is effective strategy to suppress the recombination of photo charges. They further investigated the CdS quantum dots (QDS)/vermiculite (CdS/VMT) nanocomposite that was fabricated by simply one-step method [88]. Which presented the highest hydrogen evolution rate of 92μmol h−1 under visible light (λ ≥ 420 nm) and the highest apparent quantum efficiency at 420 nm was 17.7%. As shown in figure 9(a), the color of the CdS/VMT composite films depends on the amounts of CdS QDs. 5%CdS/VMT film exhibited a multilayer structure, with a thickness of 10 μm by the doctor blade technique (figure 9(b)). Figure 9(c) illustrates the relevant processes at the photoanode of the photoelectrochemical cell leading to hydrogen generation the best ratio of 5% CdS/VMT nanocomposites.
Figure 9. (a) Digital pictures of bare VMT, 1%CdS/VMT, 5%CdS/VMT and 10%CdS/VMT on FTO/glass. (b) SEM image of the selected area shown by the red square in (a). (c) Schematic diagram of the photoelectrochemical water splitting process with the CdS/VMT hybrid photoanode. (©2014 Reproduced from [88]with permission from The Royal Society of Chemistry).
Download figure:
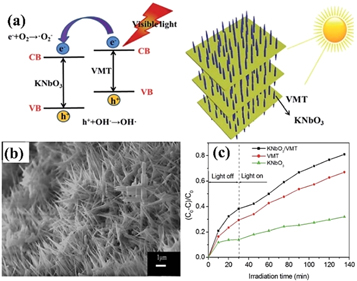
Standard image High-resolution imageApart from photocatalytic hydrogen generation, photocatalytic degradation of dye wastewater is a green, efficient and energy-saving method that can ultimately convert dyes remaining in water into CO2, H2O or other non-toxic and harmless small molecules. Typical assembly methods such as in-situ hydrothermal method, sol-gel method, electrospinning technology, have been used to fabricate VMT-based photocatalysts with unique structure. Wang et al [89] synthesized 3D potassium niobate nanoar ray/vermiculite (KNbO3/VMT) by in-situ hydrothermal method (figure 10(a)). When the reaction time was extended to 12 h, a dense nanoarray of KNbO3 needles was formed all over the surface of VMT (figure 10(d)). Figure 10(c) showed photocatalytic performance of KNbO3, VMT and KNbO3/VMT. The MB was degraded by the synergistic effect of adsorption of VMT and the photocatalytic function of KNbO3 in the composite. After 105 min visible light irradiation, the removal rate of MB in the aqueous solution could reach over 81%. Tang et al [90] used sol-gel method combined with electrospinning technology to prepare one-dimensional TiO2/VMT nanofibers with a network structure and a diameter of about 300 nm. The TiO2/VMT nanofibers annealed at 550 °C for 3 h led to the remarkable adsorption and photo-degradation ability for the treatment of methylene blue.
Figure 10. (a) The photocatalysis schematic diagram of the KNbO3/VMT composite. (b) SEM images of KNbO3/VMT samples produced with 12 h. (c) Photocatalytic activity of KNbO3, VMT and KNbO3/VMT for MB removal under visible light irradiation. The initial MB concentration was 10 mg L−1 [89]. (©2015 with permission from The Royal Society of Chemistry).
Download figure:
Standard image High-resolution image4.2. Heterogeneous catalysts
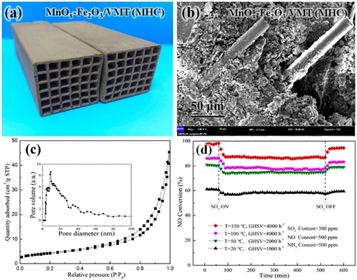
With regard to the heterogeneous catalysts, the catalytic acticity is determined by the number of active site and turnover frequency (TOF) of each site. Many efforts have been devoted to the high performance VMT-supported catalysts for various catalytic systems, such as selective catalytic reduction of NOx by ammonia (NH3-SCR), carbon oxide methanation, acetylene hydrochlorination. With the burning of fuels and the emission of exhaust gas, the quality of the atmospheric environment is deteriorating. Among atmospheric pollutants, NOx is one of the atmospheric pollutants to bring about haze, thus emission of nitrogen oxides is the currently most urgent task in China [91–93]. Selective catalytic reduction of NOx by ammonia (NH3-SCR) is an effective denitrification method [94, 95]. In 2018, Zhang and his co-workers [96] used the expanded vermiculite as a catalyst carrier to obtain groundbreaking discovery that the MnOx-Fe2O3/VMT monolithic honeycomb catalysts possessed high catalytic activity at room temperature. Meanwhile, the concept of room temperature de-NOx was proposed. The MnOx-Fe2O3/VMT monolithic honeycomb catalysts (MHCs) was prepared by extrusion (figure 11(a)). The SEM micrograph of MnOx-Fe2O3/VMT MHCs in figure 11(b) showed that the glass fibers were uniformly distributed throughout the MnOx-Fe2O3/VMT MHCs to enhance mechanical strength. The figure 11(c) presented the N2 isotherms and correspondingpore size distribution curve (inset) of a MnOx-Fe2O3/VMT MHCs, The MnOx-Fe2O3/VMT (MHCs) taken on high catalytic activity at room temperature (20 °C), The NO conversion rate was 62.2% GHSV = 1000 h−1 (gas hourly space velocity). Additionally, MnOx-Fe2O3/VMT also exhibits excellent resistance to SO2 (figure 11(d)).
Figure 11. (a) MnOx-Fe2O3/VMT MHCs: photograph of MHCs; (b) SEM image, (c) N2 isotherms and corresponding pore size distribution curve (inset). (d) SO2 resistance at various temperatures with different GHSV [96]. (Open acess).
Download figure:
Standard image High-resolution imageThe main component of natural gas is methane (CH4), which is a 'clean energy'. The preparation of synthetic natural gas by COx methanation has received extensive attention and can be used to increase the utilization of CO2[97]. In addition, the development of coal-based natural gas is also an effective way to clean coal utilization in the future [98]. Li et al [9] used expanded multilayered vermiculite (VMT) as catalyst support and synthesized NiO/VMT composite by microwave irradiation-assisted synthesis (MIAS). Compared with conventional heat treatment, the MIAS-NiO/VMT catalyst had a total CO conversion rate of 99.6%, CH4 selectivity of 93.8%, and 5.88 × 10−4 s−1 of TOF. Plasma opens new dimensions to process 2D materials because of the plasma-material interactions that promote energy transfer at the nanoscale [99–103]. Zhang et al [104] prepared a Ni catalyst system supported on two-dimensional plasma-treated vermiculite (2D-PVM-T) with a very low nickel loading of 0.5wt.% by plasma irradiation method (PIM), the CO conversion rate and TOF of PIM-Ni/PVMT reached 93% and 0.8537 s−1. Huang et al [105] prepared a VMT-surported catalyst of acetylene hydrochlorination. Compared with the traditional carbon carrier, the carbon-coated modified vermiculite (EML-VMT-C) supported mercury chloride catalytic activity can reach 97.22%. After 10 h reaction, the catalytic activity remained above 87.89%, and the carbon-coated expanded vermiculite catalyst had a catalytic selectivity of up to 100%. Therefore, the VMT-supported catalysts have broad application prospects in heterogeneous catalytic reactions.
5. Bacteria carriers and antibacterial materials
5.1. Bacteria and microbial carriers
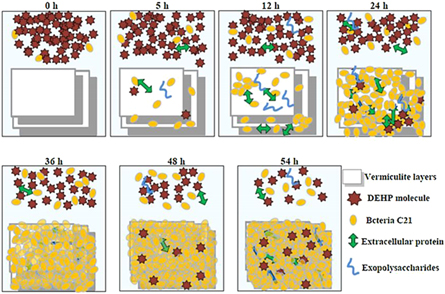
Natural mineral vermiculite is a kind of hydrated clay being rich in nutrients. It is often used as an ideal medium for microbes to study the growth of fungi. Siliva et al [106] used organic-rich vermiculite to measure the viability and predation of fungi, which provided a good environment for the growth of fungi. The mixing of rice hulls and vermiculite lead to a decrease in the dry and wet density of the matrix, available water, residual water, total porosity and moisture content, and increase of the aeration space in the substrate. It is selective for the growth of fungi [107]. Li et al [108] found that vermiculite has a good adsorption effect on Enterococcus faecalis PSCT3-7 and inhibits the growth of Salmonella typhimurium in a concentration-dependent manner. At the same time, Samlikova et al [109] prepared antibacterial chlorhexidine/vermiculite (CA/Ver), CA/Ver samples were evaluated for antibacterial activity through the minimum inhibitory concentration (MIC) against Enterococcus faecalis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Biofilm degradation materials can be prepared using fungi, Wen et al [110] formed a biofilm on the surface of vermiculite by isolating Arthrobacter strain C21 (CGMCC 7671) from constructed wetlands treated with municipal wastewater. Degradation of di-(2-ethylhexyl) phthalate (DEHP) by biofilm of strain C21 formed on vermiculite showed that DEHP was removed from the aqueous phase by biodegradation, vermiculite adsorption, and biofilm adsorption (figure 12), the synergistic effect of these three reactions increases the overall DEHP removal efficiency.
Figure 12. Synergistic effect using vermiculite as media with a bacterial biofilm of Arthrobacter sp. for biodegradation of di-(2-ethylhexyl) phthalate [110]. (© 2015, with permission from Elsevier).
Download figure:
Standard image High-resolution image5.2. Antibacterial materials
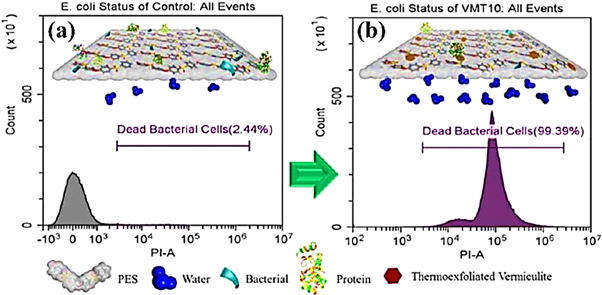
Membrane technologies have been widely used in wastewater and waste gas treatment, mixture separation and bring good social benefits. However, problems such as concentration polarization, gel plugging, even bacteria adhering to the surface of the membrane and accumulating growth in the sewage may seriously reduce the membrane separation efficiency. Modification of membranes to make them stainless has become a concern. VMT has strong water permeability and excellent antibacterial properties, so vermiculite is a good antibacterial film filler. Orooji et al [111] found that the method of mitigating biofouling of polyethersulfone (PES) ultrafiltration membrane by thermal expansion vermiculite (VMT) blending (figure 13) can scientifically solve the problem of biological pollution. Cech et al [112] studied the antibacterial properties of polyethylene (PE) nanocomposites prepared by zinc oxide nanoparticles/vermiculite nanofillers. The long-term antibacterial effect of PE nanocomposites in Gram-positive bacteria Enterococcus faecalis was determined by counting the number of colony forming units. Surfactants are also used to modify vermiculite to enhance the performance of antibacterial materials. Undakova et al [113] modified vermiculite with three concentrations of cetyltrimethylammonium (HDTMA+) to prepare organoclay mineral nanofillers in polyethylene (PE), experiments have shown that the number of bacterial colonies after 48 h is 0 CFU (colony forming unit) on the surface of PE/HDTMA+-VER nanocomposites. Hundakova et al [114] modified clay mineral vermiculite by organic surfactant cetyltrimethylammonium bromide, cerium and aluminum chloride solution to prepare PE and VT composite by melt blending, and antibacterial test results showed that PE composites had good antibacterial and antifungal effects against Staphylococcus aureus and Candida albicans.
Figure 13. Excellent biofouling alleviation of thermoexfoliated vermiculite blended poly (ether sulfone) ultrafiltration membrane [111]. (© 2017 American Chemical Society).
Download figure:
Standard image High-resolution image6. VMT-modified inorganic nanocomposites
6.1. Fire resistant materials
Fire resistant materials are inorganic materials which can use in high temperature environments. most refractory materials include the silica ingredients, and the main components of VMT is aslo silica, which means that vermiculite is a natural fire resistant materials. Expanded vermiculite is often used as an additive to introduce inorganic nanocomposites to enhance thermal stability and mechanical strength at high temperatures. Koksal et al [115] studied the properties of cement-based mortar produced from vermiculite and silica fume at room temperature and high temperature, and determined the physical, mechanical, thermal and microstructural properties of the mortar produced (figure 14). Strength and durability of cement-based mortar are increased at elevated temperatures, and the thermal conductivity is reduced to 0.257 W mK−1. The content of expanded vermiculite affects the fire resistance of the material. Chen et al [116] used three-point bending test, compressive strength test and flat plate method to determine the mechanical and thermal insulation properties and thermal conductivity of expanded vermiculite (EV)/forsterite composites before and after in-situ alumina gel modification. The results showed that when the substitution rate of the modified EV is 50% by weight, the thermal conductivity is 0.169 W m−1 K−1 (1073 K), which is increased by 30.5%. Sutcu et al [117] prepared 2.5, 5, 7.5 and 10 wt% expanded vermiculite lightweight porous clay bricks by semi-dry pressing process. The results showed that the porosity increased by 45% with the addition of vermiculite, thermal conductivity of 10 wt % expanded vermiculite sample decreased from 0.96 to 0.65 W M−1K−1. The introduction of the modified vermiculite into the inorganic nanocomposite can significantly improve the inhibition of heat transfer. Therefore, composites produced by expanding vermiculite or modified vermiculite as additives are expected to be the fire resistant materials, as presented in table 4.
Figure 14. Tests conducted: (a) flow table, (b) compressive strength, (c) elevated temperature, (d) flexural strength and (e) ultrasound pulse velocity [115]. (©2015, with permission from Elsevier).
Download figure:
Standard image High-resolution imageTable 4. Comparison of properties of thermochemical storage materials.
| Mateirals | Temperatures (°C) | Water absorption rate g g−1 | Mass energy density kWh kg−1 | Volumetric energy density kWh m−1 | References |
|---|---|---|---|---|---|
| VMT/NaNO3 | 300.9 | — | 157.2 J g−1 | — | [120] |
| VMT/Ca(OH)2 | >450 | — | 1300 kJ kg−1 | — | [123] |
| VMT/SrBr2 | <100 | 0.53 | 0.46 kWh kg−1 | 105.36 kWh m−3 | [124] |
| VMT/LiCl | <100 | 1.41 | 1.21 kWh kg−1 | 171.61 kWh m−3 | [125] |
| VMT/LiCl | 75 °C–85 °C | >0.8 | 2.6 kJ g−1 | 253 kWh m−3 | [126] |
| VMT/CaCl2 | — | 0.494 | — | 1.30 GJ m−3 | [127] |
| VMT/LiNO3 | — | 0.61 | — | 1.65 GJ m−3 | [127] |
6.2. Thermochemical storage materials
Thermal energy storage (TES) technologies could be a promising way to correct the mismatch between energy supply and demand. Methods of TES are generally divided into three types: sensible heat storage, latent heat storage [118] and thermochemical heat storage [119]. Sensible and latent thermal storage technologies have been extensively developed and used in practical solar energy storation. Compared with sensible thermal storage, latent heat has greater storage density with a much smaller temperature interval. However, leakage of phase transition process result in long-term instability of storage properties. To solve the above problems, Peng et al [120] adopted impregnation method to produce a sodium nitrate-expanded vermiculite form-stable composite phase change materials, the encapsulation of vermiculite effectively prevent the leakage during phase transition process (Organic phase change materials are described below). Unfornately, drawbacks the of determined value of phase transition temperature and low energy storage density of phase change materials restrict further application. To further enhance energy storage density and flexibility of energy utilization. thermochemical heat storage is receiving burgeoning attentions, thermochemical storage can be divided into chemical reaction (should be called 'chemical reaction without sorption' strictly) and sorption [121]. Calcium hydroxide is one of the most employed for chemical reaction storage materials, due to high volumetric energy density and impressive reversibility [122]. However, the agglomeration of Ca(OH)2 particles during the reaction hinders its further development. To solve this problem, the introduction of vermiculite not only avoid particle agglomeration, but aslo enhanced reaction performance and chemical stability [123]. Sorption heat storage, as another technology of thermochemical heat storage, not only has high heat storage density and scarcely heat loss, but also can realize the advantages of cascade utilization of heat energy across seasons. Sorption heat storage through the adsorption and desorption of adsorbents in the reversible process of desorption/adsorption is accompanied by a large amount of thermal energy storage and release.
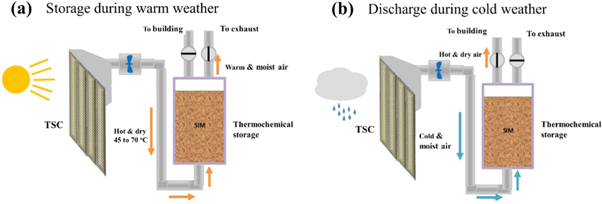
To date, the research on sorption heat storage mainly focuses on the inorganic hydration salts system. However, With water vapor as the adsorbate, the strong hygroscopic salt has a deliquescence problem, which not only causes system corrosion but also causes the material to lose heat storage, the effective method is to use a porous-rich material as an matrix to support the reaction salt. The porous matrix not only can effectively avoid the expansion and agglomeration of the reaction salt, but also rely on its surface tension to adsorb the liquid salt solution, and its large specific surface area also helps to accelerate the rate of adsorption reaction. Compared to small pore volume and weak mechanical strength of zeolite and silica gel, vermiculite known as rich porosity, high chemical stability and low cost has been widely employed as porous matrix to fabricate composite adsorbent. Zhang et al [124] developed a novel expanded vermiculite EVM/SrBr2 composite adsorbent with different salt content for low-temperature energy storage, which exhibited the water adsorption and storage density are greatly increased, the water adsorption is 0.53 g g−1, the energy storage density is 0.46 kWh kg−1, and the volumetric storage density is 105.36 kWh m−3. Zhang et al [125] and Grekova et al [126] developed composite sorbents by inserting LiCl into vermiculite for low-temperature energy storage, respectively. Inspired by the principle of sorption heat storage, Transpired solar Collector (TSC) conjuncted with suitable hydrated salt (CaCl2 and LiNO3) is an efficient devices using across seasons in thermal energy storage. As shown in figure 15, during dry warm periods (figure 15(a)), the hot air produced by the TSC can be used to chemically change the storage material and this air can then be safely discharged to the exterior of the building. During cold periods, (figure 15(b)), the cold air can be elevated in temperature by chemical reactions in the thermochemical storage and supplied directly to the building or as input air with a temperature uplift to a conventional air heating system. The hydration salt heat storage provides energy supply through hydration and dehydration but it is important to consider the rigor of the storage system of the salt, as well as the stability of the material. Porous matrix vermiculite provides stable materials and internal regions for the hydration and dehydration of vapors and inorganic salts [127]. Although sorption thermal storage systems offer some benefits, there are still critical drawbacks, such as great complexity in the system configuration (for closed systems), expensive investment, poor heat and mass transfer ability (for chemical reaction) and low heat storage density in actual systems.
Figure 15. Simplified schematic of charge and discharge stages of the thermochemical cycle using TSC for building thermal storage and heating [127] (© 2017, with permission from Elsevier).
Download figure:
Standard image High-resolution image7. VMT-modified polymer nanocomposites
The VMT-modified polymer nanocomposites consisting of a polymer matrix and VMT dispersed phase are well designed via melt intercalation, in situ polymerization, solution casting methods. To enhance the compatibility of inorganic clay VMT and polymer, In general, VMT needs to be organically treated through cation exchange between the interlayer cations of VMT and cationic surfactant. Recently, large quantities of VMT-modified polymer nanocomposites have been prepared by various synthetic methods. Given their diverse applications, they all can be categorized into six categories. In this part, we systematically introduce all aspects of the application and potential of VMT-modified polymer nanocomposites.
7.1. Mechanical nanocomposties
Thermal and mechanical properties are important properties of polymeric materials. The introduction of vermiculite not only can effectively improve the thermal and mechanical properties of polymer materials, but also increase the tensile modulus and impact strength of nanocomposites [128, 129] Surfactants have been widely employed to increase the spacing of the vermiculite, improving the mechanical and thermal properties of the composite [130]. Isci et al [131] figured out the impact of vermiculite and its organoclays, which were decorated with cationic and anionic surfactants polyvinylbutyral nanocomposites via solution casting method, which showed that the organovermiculite prepared with anionic surfactant possessed higher elastic modulus and the melting temperatures. Nevertheless, the further development of mechanical nanocomposties is largely constrained by the agglomerate and poor dispersion of in polymer matrix with higher content of VMT. To further improve the dispersion of VMT with better homogeneity, Zhang et al [132] adopted the ultrasonic in situ polymerization to prepare organically modified vermiculite (OVMT) polymethylmethacrylate (PMMA) nanocomposites with good homogeneous dispersion and strong interfacial interaction of OVMT in PMMA, Which showed better mechanical and thermal properties than those prepared without the assistance of ultrasonic irradiation, as presented in table 5.
Table 5. The mechanical properties of VMT-modified polymer nanocomposties.
| Nanocomposites | Methods | Tensile modulus (GPa) | Tensile strength (MPa) | The increased rates of tensile strength (%) | Strain at break (%) | Izod impact strength | References |
|---|---|---|---|---|---|---|---|
| PLLA | — | 1.70 ± 0.02 | 73.52 ± 2.05 | 6.04 ± 0.2 | — | 9.26 ± 0.24 | [128] |
| PLLA/VMT10 | melt blending | 2.00 ± 0.13 | 66.17 ± 1.17 | 4.54 ± 0.4 | — | 11.56 ± 1.39 | [128] |
| EA | — | — | 7.3 ± 0.4 | — | 15 ± 0.5 | 5.5 ± 0.5 | [129] |
| 2BTPC-VMT | intercalate | — | 13.5 ± 0.6 | — | 9 ± 0.5 | 9.1 ± 0.4 | [129] |
| PUCPB | — | — | 17.6 | — | 337 | — | [130] |
| PUCPB/VMT | intercalating polymerization | — | 19.8 | 12.5 | 359 | — | [130] |
| PUCPB/OVMT | intercalating polymerization | — | 26.8 | 52.3 | 443 | — | [130] |
| PVB | — | ∼1.58 | — | — | — | — | [131] |
| 2Vk+PVB | Solution casting method | ∼2.81 | — | — | — | — | [131] |
| PMMA | Ultrasonic in situ polymerization | 0.964 ± 0.23 | 56 ± 2 | — | 8.3 ± 0.6 | — | [132] |
| PBTP-S3 | Ultrasonic in situ polymerization | 1.177 ± 0.026 | 65 ± 4 | — | 14 ± 1.8 | — | [132] |
7.2. Flame-retardant nanocomposties
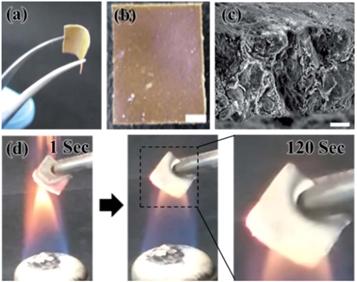
Vermiculite possesses inherent heat insulating and stability of physical and chemistry withstanding a flame of about 1200 °C. Therefore, vermiculite as a filler have been reported to reinforce flame-retardant property of composites. These flame-retardant nanocomposites are required via modified polymer with vermiculite or to construct novel film with nanosheets of others. The effect of flame-retardant nanocomposites based on polyers was mainly evaluated by limited oxygen index, thermogravimetric analysis and carbonization rate under actual fire [133]. Wang et al [134] manufactured the new flame-retardant plywood by adding expanded vermiculite (EVMT) in the adhesive and by surface treatment as a flame-retardant coating, and the results showed that EVMT increased the limited oxygen index value of the treated sample and reduced the thermal degradation energy. Xue et al [135] significantly improved the fire protection and carbonization structure of the coating by adding 7% expanded vermiculite (mass) to the coating. The EV sheet structure promoted the expansion and carbonization of the coating under fire. The thermal insulation properties of the layer reduce its corrosion performance at high temperatures. Yan et al [136] also confirmed that vermiculite filling improves the performance of flame retardant composites, and that the limiting oxygen index of polyimide foam increases from 31 to 33.5% as the vermiculite increases from 0 to 8%. The peak heat release rate is reduced. Cheong et al [137] prepared the vermiculite–PEG (polyethylene glycol) hybrid film and the concentration of vermiculite was about 75 wt%. As shown in figure 16(a), the vermiculite particles were well-dispersed in the cross-linked PEG network, allowing the vermiculite–PEG hybrid film also to be flexible, color of vermiculite–PEG hybrid films was observed (figure 16(b)). The SEM image of the cross-sectional view of the vermiculite–PEG hybrid film exhibited approximate thickness (figure 16(c)), It was found that in the absence of vermiculite, the polymer film was immediately burned off upon contact with the flame. At a vermiculite concentration of about 75 wt%, a 20 mm thick film could withstand an actual fire of more than one minute (figure 16(d)). Moreover, the film manufacturing process is simple, time efficient, and thickness controllable.
Figure 16. (a) Photographic images of (A) the fabricated (S3) with its flexibility and (b) Photographic images of 75 wt% vermiculite–PEG hybrid film. (c) SEM image of the cross-sectional view of the vermiculite–PEG hybrid film (d) Photographic images of fire endurance tests of the vermiculite–PEG hybridfilms. S3 (thickness: 60 mm, 1 s and 120 s after ignition) [137]. (©2015 with permission from The Royal Society of Chemistry).
Download figure:
Standard image High-resolution imageVermiculite is an anionic clay that theoretically forms new materials with cationic materials through molecular self-assembly techniques. In order to improve the flammability and gas barrier properties of cellulose-based films, scientists have used a layer-by-layer deposition process to form highly-arranged clay nanosheets and cellulose nanofibers together to form thin films like a nano-brick wall structure. The nanobrick wall structure exhibits high flame resistance and super oxygen barrier [138].
7.3. Phase change materials
Phase change material (PCM)-based thermal energy storage systems have been recognized as one of the most advanced energy technologies for improving energy efficiency and sustainability, since the phase change material has a constant temperature characteristic in the phase change endothermic/exothermic process [139, 140]. The single inorganic or organic phase change materials are obsessed by the leakge of PCMs making the declined of storage capacity as well as the index of security to constrain further application for buildings. Therefore, the development of composite phase change materials has become a hot research topic in the field of heat storage materials. Expanded multilayered VMT has emerged as a promising matrix material of PCMs due to the high porosity and low densities as well as flame-retardant property to overcome this disadvantage. Consequently, a array of VMT–based composite PCMs have been sucessfully engineered, such as composite PCMs of paraffin/expanded vermiculite [141, 142], Lauric acid (LA)/expanded vermiculite [143]. Aforesaid composite PCMs exhibit excellent thermal durability and stability, Nevertheless, phase change temperature high for low-temperature thermal energy storage of one eutectic composite PCMs is contradictory, meanwhile, issue associated with low thermal conductivity has accompanied the introduction of VMT. To solve the above problems, the binary or ternary eutectic mixture can effectively modulate the phase change temperature. Due to high chemical stability and good compatibility with PCMs, the introduction of carbon-based materials not only significantly enhance thermal conductivity of composite PCMs, but also further prevent the leakge of PCMs [144, 145]. Wen et al [146] reported that fatty acid eutectic (citric acid and lauric acid) was impregnated into expanded vermiculite (EVM) to form composite PCMs, The differential scanning calorimeter (DSC) result showed that the melting temperatures and latent heat values of the PCMs are in the range of about 21 °C–23 °C and 81–117 J g−1. In addition, the thermal conductivity of CA-LA/EVM was 0.135 W m−1 K for 5 wt% of expanded graphite (EG), which was an increase of 87.41% compared to pure composite PCMs. Besides, Zhang et al [147] embedded ternary eutectic mixture of lauric acid-palmitic acid-stearic acid (LA-PA-SA) into the interlayer of porous vermiculite to obtain ternary eutectic mixture/vermiculite composite PCMs. The thermal cycle of the LA-PA-SA ternary eutectic mixture/vermiculite inappreciably change after 1000 cycles, and the thermal conductivity improved by 68% for 2 wt% expanded graphite in the PCMs compared to LA-PA-SA/VMT. Furthemore, the introduction of inorganic compound silicon carbide nanowires (SiC NWs) can improve the stability and thermal conductivity of polyethylene glycol-enwrapped silicon carbide nanowires network/expanded vermiculite composite phase change material [148]. The above results indicate that the eutectic mixture/vermiculite composite PCMs possess good thermal stability and thermal conductivity.
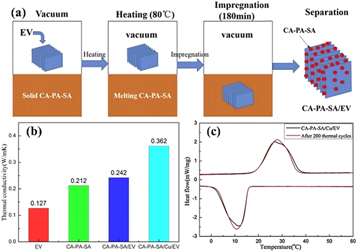
Aparet form carbon-based material, metals and metal oxides have been widely investigated as filler for enhancing the thermal conductivity and latent heat of composite PCMs. Deng et al [149] reported polyethylene glycolsilver nanowires (Ag NW) as thermal conductive reinforcing fillers, expanded vermiculite (EVM) as a supporting material to prepare polyethylene glycol silver nanowires/EVM phase change material to overcome the liquid phase leakage during the phase change process and improve the thermal conductivity of the PEG. The eutectic of perovskite-type stearic acid (CA-PA-SA) can also be incorporated into expanded vermiculite (EV) to prepare form-stable phase change material (figure 17(a)). As shown in figure 17(b), the thermal conductivity values of EV, CA–PA–SA, CA–PA–SA/EV, and CA–PA–SA/Cu/EV are 0.127 W mK−1, 0.212 W mK−1, 0.242 W mK−1, and 0.362 W mK−1, respectively. With 5 wt% Cu powder added, the thermal conductivity of the composite increased by approximately 49.58%. According to the differential scanning calorimetry (DSC) curve (figure 17(c)). The melting and freezing temperatures of CA–PA–SA/Cu/EV before thermal cycles are 19.4 °C and 15.6 °C, respectively. After thermal cycles, these temperatures are 19.2 °C and 15.6 °C, respectively. The latent heats of melting and freezing vary from 119.2 J g−1 and 116.1 J g−1 to 115.6 J g−1 and 113.8 J g−1, respectively. The melting and freezing temperature and latent heat values of the CA-PA-SA/Cu/EV after thermal cycling slightly change at a reasonable level. Therefore, the prepared CA–PA–SA/Cu/EV composite PCM exhibited good thermal reliability after thermal cycling [150]. Wei et al [151] used Al2O3-loaded expanded vermiculite (AEV) as a support matrix to prepare lauric acid-stearic acid eutectic mixture lauric-myristi-stearic(LA-MA-SA) acid/Al2O3-loaded expanded vermiculite composite phase change material. The thermal conductivity of LA-MA-SA/aEV/AO was 0.671 W mK−1, which was 37.5% higher than LA-MA-SA/AEV. Similarly, the addition of titanium dioxide improves thermal conductivity and latent heat of vermiculite-based composite PCMs [152]. Therefore, phase change materials prepared on the basis of expanded vermiculite have great potential in thermal storage systems for energy-efficient buildings, as presented in table 6.
Figure 17. (a) Schematic of a vacuum impregnation system for synthesizing CA–PA–SA/EV composite PCMs. (b) Thermal conductivities of EV, CA–PA–SA, and composite PCM. (c) DSC curves of CA–PA–SA/Cu/EV [150]. (© 2018, with permission from Elsevier).
Download figure:
Standard image High-resolution imageTable 6. Comparison of the thermal properties reported in previous references.
| Composite PCMs | Thermal conductivity (W mK−1) | Mass ratio of heat storage material (%) | Melting point (°C) | Melting enthalpy (J g−1) | Freezing point (°C) | Freezing enthalpy (J g−1) | References |
|---|---|---|---|---|---|---|---|
| Paraffin/EVM-800 | — | — | 27.0 ± 0.1 | 77.6 ± 4.3 | 25.1 ± 0.4 | 71.5 ± 5.9 | [141] |
| Octadecane/VMT | 0.1569 | 80.65 | 26.1 | 142.0 | 24.9 | 126.5 | [142] |
| LA/EVM | 0.49 | — | 41.88 | 126.8 | 39.89 | 125.6 | [143] |
| EVMCP | 0.452 | 53.2 | 53.1 | 103 | 48.85 | 101.14 | [144] |
| SA/EVCss-CPCMs | 0.52 | 63.12 | 67.12 ± 1.33 | 134.31 ± 3.05 | 66.16 ± 0.49 | 135.94 ± 2.53 | [145] |
| CA-LA/EVM | 0.253 | 57.48 | 23.61 | 81.34 | 20.93 | 79.30 | [146] |
| LA-PA-SA/VMT | 0.56 | 50 | 31.4 | 75.8 | 30.3 | 73.2 | [147] |
| LA-PA-SA/VMT/EG | 0.94 | 50.2 | 30.6 | 72.7 | 30.4 | 68.8 | [147] |
| PSE3.29 | 0.53 | 73.12 | 51.87 | 64.93 | 24.37 | 60.48 | [148] |
| PEG-Ag/EVM-ss-CPCM19.3 | 0.68 | 58.8 | 59.97 ± 0.15 | 99.1 ± 2.6 | 43.51 ± 0.15 | 96.4 ± 2.9 | [149] |
| CA-PA-SA/EV | 0.242 | 70 | 19.3 | 117.6 | 17.1 | 118.3 | [150] |
| CA-PA-SA/Cu/EV | 0.362 | — | 19.4 | 119.2 | 15.6 | 116.1 | [150] |
| LA-MA-SA/aEV/AO | 0.671 | — | 28.6 | 113.7 | 26.9 | 108.5 | [151] |
| SA/aEVT | 0.58 | — | 65.9 | 146.8 | 63.4 | 141.7 | [152] |
7.4. Water absorbent ploymers with slow-release fertilizer
The water-retaining slow-release fertilizer can adjust the water adsorption ratio of the fertilizer according to different crops, soil, climate and other conditions, and is widely used in food crops, cash crops, horticultural plants and the like. The water-retaining slow-release fertilizer combines the fertilizer with the superabsorbent resin composite material, which can not only absorb water, retain water, resist drought and protect the sorghum, but also slowly supply the nutrient slow-release fertilizer function to the crop. Yuan et al [153] reported that poly(acrylic acid-co-acryla mide)/sodium carboxymethyl cellulose/vermiculite superabsorbent polymers (SAPs), embedding urea into their networks to obtain slow release fertilizer (SSRF) SAPs, The addition of urea not only gave SAPS a sustained release of urea (more than 30 days in the soil), but also increased its water adsorption (17.4%). In 2017, Ma et al [154] prepared the polyacrylamide copolymer (PAMVT) microspheres by inverse emulsion polymerization using modified vermiculite as monomer. The experimental results showed that the water adsorption capacity can reach 80.2%, water flooding recovery increased from 69.33 to 77.56%. Li et al [155] studied the use of vermiculite for partial replacement of sulphoaluminate cement (SAC) to prepare a cementitious material with enhanced water absorption capacity and fertilizer retention properties, This is essential for plant growth in a cement environment.
7.5. Modified asphalt with good long-term aging resistance
The durability of asphalt is the most important factor affecting the quality and longevity of asphalt pavement. The aging of asphalt pavement is a long-term process. Therefore, improving the performance of asphalt and preparing asphalt mixture with lower thermal conductivity and improving its anti-aging ability have practical significance. Ma et al [156] found that the thermal conductivity was reduced by 40% after the addition of 10% vermiculite powder in the asphalt mixture, and the internal temperature of the asphalt mixture was reduced by 2 °C. Furthermore, the combination of 3% organically expanded vermiculite and warm asphalt was added. The material has more significant anti-aging properties [157]. Bitumen with ZnO nanoparticles and expanded vermiculite exhibits good long-term aging resistance [158]. Zhang et al [159] also confirmed that the composite of nano-ZnO and OEVMT can effectively improve the performance of SBR modified asphalt to reduce thermal oxidation and photo-oxidative aging. Similarly, In addition, the improvement effect of the anti-aging modifier is not only related to the degree of aging of the asphalt, but also depends on the nature of the asphalt. Zhu et al [160] also found that modified asphalts containing surface-modified nano zinc oxide and organic expanded vermiculite showed lower composite modulus and higher phase angle, indicating that it has good thermal oxidation and light aging resistance.
7.6. Vermiculite-modified bionanocomposites
Plastic products, as one of the three major artificial materials, are inseparable from owing to their low production cost, light weight and good stability, which potentially manufacture a considerable amount of plastic garbage and bring hidden dangers to the environment. Recently, scientists from all over the world have surprisingly reported the existence of plastic particles found in human feces, which means that plastic waste will not only cause white pollution, but also leads to a panic in plastic eating. Tremendous researches therefore focus on several biodegradable and biosourced single polymer material. However, these conventional single polyer materials with biodegradable ability suffer from thermal instability, low rate of crystallization, lower viscosity [161], which prevent their use in practical packaging industry. Fortunately, The introduction of VMT not only effectively reduce the crystallinity of the polymer but also enhance the biodegradability of the nanocomposite [162]. In 2017, Reis et al [163] developed the poly (3-hydroxybutyrate-co-3-hydroxy-
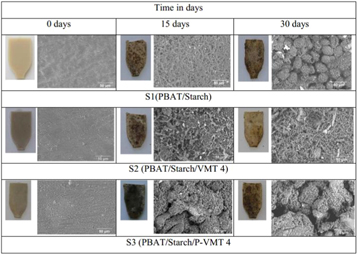
valerate) biocomposite of natural vermiculite and modified vermiculite by melt intercalation technique. Evaluation of thermal behavior, biodegradation, and food packaging interactions found that biocomposites with VMT have better thermal stability and high biodegradability and inert when in contact with food. As such, biodegradable biocomposites are currently the most perfect beverage and food packaging materials in the world [164]. Oliveira et al [165] prepared the degradable composites based on polyester and starch matrix with vermiculite by the melt intercalation technique, followed by injection molding. Unmodified VMT and VMT organically modified with tetrabutyl phosphonium bromide were used in each composition. Visual analysis of all specimens after 15 and 30 days of burial in simulated soil showed dark spots distributed in different regions on the surface of the material, suggesting degradation by the action of microorganisms (figure 18). The SEM images (figure 18) indicates that after burial in simulated soil for 15 and 30 days degradation caused irregular surfaces containing grooves, small craters and lighter regions caused by the activity of microorganisms through biofilm formation. Addition of VMT and cetyltributylphosphonium bromide (P-VMT) to polybutylene adipate-terephthalate and starch blends increases crystallization temperature (TC) and crystal melting temperature (TM) [166], Fernandez et al [167] found that the erosion mechanism of the polymer matrix occurs at the surface of the membrane and begins at the interface of the polymer and clay minerals. For vermiculite L-lactic acid (PLLA) composites, the type and dispersion of clay minerals greatly affects the degradation rate of PLLA.
Figure 18. Visual analysis of pure matrix and its composites and SEM micrographs of sample surfaces [165]. (© 2015, with permission from Elsevier).
Download figure:
Standard image High-resolution image8. VMT-derived nanomaterials
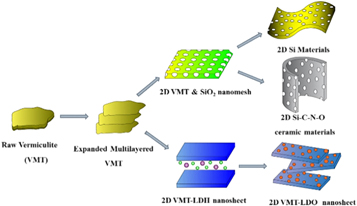
VMT-derived nanomaterials represent an emerging class of VMT-based nanomatetials. many Efforts have been made to engineer vaious novel materials (figure 19) to expand its application ranges, such as 2D silica nanomesh, 2D VMT-based layered double hydroxide (LDH), lithium silicates, silicon carbide ceramic material. Hence, the objective of this section predominately summarize the up-to-date progress about VMT-derived nanomaterials with particular emphasis potential application in various fields.
Figure 19. Vermiculite-derived emerging nanomaterials.
Download figure:
Standard image High-resolution image8.1. Two-dimensional SiO2 and silicon carbide ceramic nanomaterials
VMT, as a 2D layered silicate mineral, is used to retain the lamellar SiO2 skeleton structure and completely dissolve the metal components by acid etching to obtain layered porous SiO2 nanomaterials. Wang et al [168] prepared mesoporous silica nanosheets (SiNSs) by acid-treated VMT (originally Verm), as-prepared SiNSs exhibited broad prospect in catalyst carriers, sorbents and templating fields. Based on the rich VMT mineral resources in Xinjiang, Dan et al [28] succeeded in the preparation of 2D vermiculite-based SiO2 (VMT-SiO2) nanomesh with a thickness of about 5.5 nm, specific surface area of 505 m2 g−1, average pore diameter of 1.8 nm and a pore volume of 0.406 cm3 g−1. 2D VMT-SiO2 nanomesh is employed as catalyst carrier for COx methanation [169], Compared to 3D mesoporous silica (MCM-41) molecular sieves carrier, the active component Ni particles was anchored to 2D VMT-SiO2 to prevent the active component from agglomeration sintering during the methanation reaction. Consequently, NiO/2D VMT-SiO2 exhibited CO conversion of 85.9%, CH4 selectivity of 78%, 0.1151 s−1 TOF, which were much better than those measured for NiO/MCM-41. Song et al [31] successfully fabricated porous silicon carbide-supported nickel (Ni/VMT-SiC) as a COx methanation catalyst using two-dimensional mineral vermiculite. Since the SiC carrier has excellent thermal conductivity, the formation of 'hot spots' in the catalyst bed can be effectively avoided, and the active component of the catalyst Ni/VMT-SiC has high dispersibility and small particle size interacts with a strong metal carrier, and the Ni/VMT-SiC catalyst exhibits good catalytic activity at low temperatures. At 250 °C and 0.1 MPa, the CO conversion rate can reach 83.2%, and there is almost no attenuation after 50 h cycle, which has good stability. Unfortunately, 2D VMT-SiO2 is only used as supported catalysts. Bearing this in mind, we believe that 2D VMT-SiO2 still have many potentials, such as photocatalysis, adsorption, andfire resistant aspects, to be further explored.
8.2. Two-dimensional MgAl layered double hydroxide
Magnesium aluminum hydrotalcite compound is a new type of inorganic functional material with 2D anionic layered structure. The layered compound is a kind of compound with layered structure and interlayer ion with exchange ability. The intercalation and interlayer ion of the layered compound under the action of the strong polar molecule can be used. In exchange, some functional guest substances are introduced into interlayer voids and the distance between laminates is stretched to form layer column compounds. Hydrotalcite has unique structural characteristics, which can be used as basic catalyst, redox catalyst and catalyst carrier. For example, it can be used as a catalyst for hydrogenation, reforming, cracking, condensation and polymerization [97].
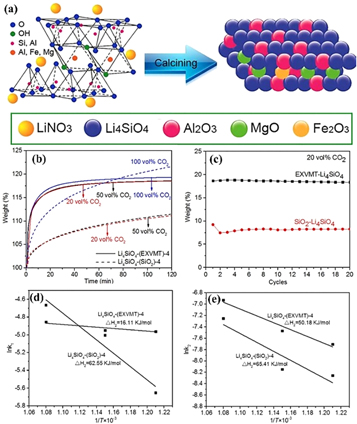
In 2017, Li et al [29] synthesized LDH using the spent liquor after mixed-acid etching of VMT, which mainly contained Mg2+ and Al3+. The as-calcined layered double oxide (LDO) was used as a catalyst support for CO methanation. VMT-LDO as a catalyst support provided a superior position for implantation of NiO species and the as-obtained catalyst exhibited excellent performance. It exhibited excellent catalytic performance over the whole temperature range of 250–500oC. Ni/VMT-LDO achieved the best activity with 87.88% CO conversion, 89.97% CH4 selectivity, and 12.47 × 10−2 s−1 TOF at 400 oC under a gas hourly space velocity of 20,000 mL g−1 h−1. This study demonstrated that VMT-LDO as a catalyst support provided an efficient way to develop high-performance catalysts for synthetic natural gas (SNG) from syngas. Zhang et al [150] also prepared VMT-LDH (ie, MgAl-CO3LDH in [170]), the mixed oxide from VMT-LDH as an intermediate and high temperature CO2 adsorbent. This demonstrates a unique and environmentally friendly solution for obtaining two carbon dioxide sorbents from inexpensive raw materials, an idea that is valid for the efficient use of other minerals.
8.3. Li4SiO4 nanoparticles
With the increase of CO2 emissions and the intensification of the greenhouse effect, capturing CO2 directly from the air is a hot spot for researchers in recent years. Considering the economic cost and energy consumption factors, after the liquid ammonia, the solid adsorbent is on the stage of CO2 capture [171]. Compared with other sorbents, Li4SiO4 is considered to be a promising sorbent due to its ability to adsorb CO2 in a wider range of temperature, the adsorption capacity is larger, the desorption temperature is lower, and the adsorption/desorption cycle is stable. Zhang et al [30] prepared a novel Li4SiO4-based high-temperature CO2 sorbent (figure 20(a)) directly from the expanded vermiculite (EXVMT) for the first time. Due to better dispersion and higher porosity of Li4SiO4-(EXVMT)-4, which presented much higher CO2 sorption capacity and faster sorption rate at relatively lower sorption temperature (550 °C) and enhanced CO2 sorption/desorption cycling stability (figure 20(c)). Li4SiO4-(EXVMT)-4 impurity phases prevent the sintering and thus increase the cycling stability. Furthermore, this sorbent was demonstrated lower activation energies for both the chemisorption and the diffusion processes (figures 20(d), (e)). By decreasing the CO2 concentration from 100 to 20 vol%, the CO2 sorption capacity of Li4SiO4-(EXVMT)-4 only slightly decreased from 19.4 to 18.6 wt% (figure 20(b)), however, the capacity for Li4SiO4 synthesized from pure SiO2 decreased significantly from 22.0 to 11.3 wt%. On the basis of previous work, Zhang et al [172] prepared hierarchical Li4SiO4 nanoparticle/flakers composite through VMT/MCM-41 hybrid precursor, and proved that this novel Li4SiO4 possesses the advantages of MCM-41-Li4SiO4 and VMT- Li4SiO4, including high CO2 adsorption, less affected by CO2 concentration, fast CO2 capture kinetics and excellent cycle performance.
Figure 20. (a) Schematic illustration of the synthesis of Li4SiO4 from vermiculite. (b) CO2 sorption isotherms of Li4SiO4-(EXVMT)-4 and Li4SiO4-(SiO2)-4 sorbents under different CO2 concentrations at 550 °C. (c) CO2 sorption/desorption cycling performance of optimized Li-(EXVMT)-4 and Li-(SiO2)-4 sorbents. (d), (e) Plots of lnk versus 1/T for the two different processes of chemisorption (k1) and diffusion (k2) on Li4SiO4-(EXVMT)-4 and Li4SiO4-(SiO2)-4 sorbents [30] (© 2018, with permission from Elsevier).
Download figure:
Standard image High-resolution image9. Conclusion and outlook
In summary, with the joint efforts of researchers at home and abroad, a considerable number of vermiculite-based functional nanomaterials have been successfully prepared and have shown great potential prospects in people living needs and industrial production. In terms of environmental adsorption materials, vermiculite base functional materials have achieved a lot of fruitful researches in wastewater treatment of heavy metals, dyes, drugs, etc. However, there is still room for improvement in aspects of selective adsorption, rapid adsorption, increased adsorption capacity, and number of recovery etc. In terms of supported catalysts, expanded vermiculite is an excellent catalyst carrier, rich in elements such as magnesium aluminum silicon and unique 2D nano-sized structure, high temperature stability, especially in the application of photocatalytic degradation of dyes, vermiculite The synergistic effects of adsorption and catalysis can bring attractive characteristics or functions, so they are widely used in the fields of photocatalysis and heterogeneous catalysis.
For inorganic and inorganic composites, expanded vermiculite or modified vermiculite is often introduced as an additive into inorganic materials to improve fire resistance and thermochemical storage and to enhance t-hermal stability and mechanical strength at elevated temperatures. In the aspect of organic and inorganic compounding, the expanded vermiculite is blended into the polymer to prepare composite materials such as flame retardant, phase change stability, antibacterial and biodegradable, to strengthen the tensile modulus, impact strength, thermal insulation and easy degradation of a single polymer, and greatly improve the shortcomings of leakage and low thermal conductivity during phase transformation of phase change materials. while facing the problems of how to achieve the establishment of the VMT-based composite material industry chain and large-scale production.
It is noteworthy that nanomaterials derived from VMT such as 2D SiO2 nanomesh, silicon carbide ceramic nanomaterials, Li4SiO4 nanoparticles and MgAl-LDH are in their infancy. Therefore, we believe that nanomaterials derived from VMT still have many potentials, such as Si-C-N-O ceramic materials, fire resistant materials, CO2 capture material, silicon cathode electrode materials and so on, to be further explored. More importantly, research on VMT elaborated processing techniques, such as intercalation and exfoliation, and should be intensified to form basic unit nanosheets to construct novel material with nanosheets of others.
Acknowledgments
The work was supported by Major Scientific and Technological Project of Bingtuan (No.2018AA002), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_15R46).
Competing financial interests
The authors declare no conflicts of interests.