Abstract
The degradable polymer biomaterials have great potential in the field of nerve regeneration, which have gradually been applied to spinal cord injury repair. In this study, a polylactic acid-glycolic acid copolymer (PLGA) porous scaffold was prepared by the process of phase inversion, then the surface of the scaffold was modified by polydopamine (PDA) as a substrate, followed by adsorption of nerve growth factor (NGF) to obtain a PDA-PLGA/NGF scaffold. The characterization and hydrophilicity of the scaffolds were evaluated by electron microscopy, EDX and contact angle measurement. The effect of PDA modification on the binding efficiency and release profile of NGF was observed by ELISA. The proliferation and differentiation of NSCs on the surface of biomaterials was evaluated by MTT, immunofluorescence staining and PCR. Subsequently, the rat T9 spinal cord transection model was established and the nerve scaffolds were implanted to injured site. Our results showed PDA modification could significantly improve the NGF adsorption capacity and provide sustained release of NGF. PDA-PLGA/NGF scaffolds could not only enhance NSCs proliferation and neuronal differentiation in vitro, but also promote recovery of spinal cord injury in vivo. Therefore, we believed that PDA-PLGA/NGF scaffolds could be a promising method to facilitate neurogenesis and repair spinal cord injury.
Export citation and abstract BibTeX RIS
1. Introduction
Spinal cord injury (SCI) can lead to severe motor/sensory dysfunction below the injury, and the treatment of SCI is a global medical problem [1]. Traditional treatment methods, such as methylprednisolone shock and surgical treatment, have some shortcomings. With the rapid development of tissue engineering, the transplantation of biomaterials combined with drugs or growth factors in the injury site has attracted substantial attention [2–6]. Polylactic acid (PLA), polycaprolactone (PCL), and poly(lactide-glycolide acid) (PLGA) have low immunogenicity, toxicity and degradation rates, as well as adjustable mechanical properties, which make these materials applicable in the field of tissue engineering. Among commonly used biodegradable materials, the pure PLGA scaffold has been confirmed to improve motor function in rats after SCI to some extent [7]. However, PLGA has no biological activity, and the surface is hydrophobic, which limits its nerve repair effect. Therefore, it is important to incorporate nerve-related growth factors into PLGA to achieve neurogenesis effects. Nerve growth factor (NGF) can not only effectively promote the proliferation and neuronal differentiation of NSCs but also have a clear neuroprotective effect. Injecting NGF to rats after SCI can promote motor function [8, 9], so combining NGF with PLGA will impart biological nerve repair activity on to the pure PLGA scaffold. However, the traditional direct mixing of PLGA and NGF to prepare a nerve scaffold requires a large amount of organic solvent, which can inactivate and damage proteins, including growth factors. Therefore, polydopamine was chosen as an adhesive to adsorb NGF on the PLGA scaffolds.
In recent years, inspired by mussel adhesion proteins, many studies have proven that polydopamine (PDA) is structurally similar to adhesion proteins, and the functional groups of catechol and lysine in PDA also impart superior adhesion. Under basic conditions, the catechol functional groups of PDA can undergo oxidative self-polymerization and form a polydopamine coating by covalent or non-covalent bonding on the surface of polymers, metals, etc [10, 11].
To date, many studies have employed PDA for modifying materials. Ku et al [12] found that the hydrophilicity of PCL fibres was greatly improved by modification with polydopamine, which allowed the hydrophilic properties of hydrophobic materials such as PLGA to be improved. Wang ZL et al [13] used PDA to modify the surface of materials and used it to adsorb peptides related to bone formation. The results showed that cell behaviours such as cell adhesion, proliferation and osteogenic differentiation were greatly improved by this modification. Compared with the surface modification methods for polymeric materials such as surface activation, illumination, and chemical corrosion, PDA coating is a relatively simple and convenient method for material modification for imparting bioactivity, and it does not affect the internal structure and mechanical strength of the material [14, 15], giving it great potential for clinical applications in various fields.
In this study, a degradable, porous PLGA scaffold was prepared by the phase inversion method, and the surface of the scaffold was modified by polydopamine to physically adsorb NGF. The material properties of the scaffolds were evaluated. The effects of the PDA-modified scaffolds on the proliferation and differentiation of NSCs were tested. Then, a rat T9 spinal cord transection model was established, and the lesion site was transplanted by the scaffold. The BBB (Basso, Beattie, and Bresnahan) score was determined and tissue staining was performed to evaluate motor function and pathological changes in the spinal cord after scaffold implantation. This experiment will provide a theoretical basis for the application of PDA-modified scaffolds in SCI.
2. Materials and methods
2.1. Materials
Poly(lactide-co-glycolide) (PLGA, Mw = 40,000 g. mol−1) with a lactic acid–glycolic acid ratio of 80:20 was purchased from Changchun SinoBiomaterials Co., Ltd Recombinant Human NGF was purchased from Peprotech. Dichloromethane (DCM) was obtained from Beijing Chemical Works. 3-Hydroxytyramine (Dopamine) hydrochloride was purchased from Adamas Reagent Co. Ltd The main components of the NSC culture medium (B27, bFGF, and EGF) were purchased from Gibco-Invitrogen.
2.2. Manufacture of the PLGA scaffolds and surface modification
The manufacture of the PLGA scaffolds was followed as previously described [16]. In brief, the PLGA solution was prepared by dissolving 1.0 g of PLGA in 5 ml of NMP. After magnetic stirring at room temperature for 24 h, (NaCl) particulates of 250–450 μm in diameter were added into the PLGA/NMP mixture in an internal mixer at 60 rpm for 5 min. The weight ratio of the salt particulates to PLGA/HA was 6: 1. The mixture was immersed in deionized water for 2 days to thoroughly remove the organic solvent, and then the prepared material was freeze-dried and transferred to a vacuum desiccator for storage. Then the polymer scaffold was trimmed into a rod shape containing small channels parallel to its longitudinal axis. Subsequently, dopamine hydrochloride was added to a 10 mM solution of Tris-HCl (pH = 8.5) to produce a dopamine solution with a concentration of 2 mg ml−1. The dried scaffolds were completely immersed in the dopamine solution and continuously shaken at room temperature for 3 h. After the oxidative polymerization of dopamine was complete, the PDA-PLGA scaffold was removed and washed with deionized water 5 times to completely remove unreacted dopamine molecules. The reaction scheme was shown as figure 1.
Figure 1. The reaction scheme for PDA-PLGA scaffold.
Download figure:
Standard image High-resolution image2.3. Characterization
The surface morphologies of the PLGA scaffolds and PDA-PLGA scaffolds were observed using a scanning electron microscope (SEM, XL30 FEG, Philips). Energy-dispersive x-ray spectroscopy (EDX, Philips, XL-30W/TMP, Japan) was performed to determine the elemental composition of the porous scaffolds. To analyse the surface hydrophobicity, the water contact angles were measured to evaluate the surface wettability using the sessile drop method on a contact angle system (VCA 2000, AST).
2.4. Immobilization and determination of NGF on scaffolds
The pure PLGA scaffolds and the PDA-PLGA scaffolds were immersed in 1 ml of NGF solution (200 ng ml−1). The supernatants were collected, and the scaffolds were then cleaned with distilled water three times. The washing liquid was collected and mixed with the previous supernatants. The amount of NGF in the collected mixture and the release profiles of the NGF were evaluated with an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions using a microplate reader (Tecan Infinite M200). All experiments were performed in triplicate, and qualitative values are expressed as the average and standard deviation.
2.5. Neural stem cell culture
Neural stem cells were isolated from the cerebral cortex of embryonic mice (E11.5). The dissociated cells were cultured and purified at a density of 50,000 cells cm−2 in T25 culture flasks (Corning, NY, USA) and incubated at 37 °C in a 5% CO2 humidified incubator. One millilitre of medium was added to the culture flasks every 2 days. NSCs were passaged every 5 days, and cells between passages 2 and 6 were used for experiments.
2.6. Cell proliferation assays
NSC proliferation on various scaffolds was assayed using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) method. In brief, the porous scaffolds were sterilized by immersion in 70% alcohol for 30 min. After being washed with PBS three times, the samples were placed in 24-well tissue culture plates. Then, 1 × 104 NSCs were seeded into each well, and the samples were incubated at 37 °C under 5% CO2 for 1, 3, and 5 days. Then, 50 μl of MTT (Sigma) stock solution in PBS was added to each well, and the cells were incubated at 37 °C for 4 h. Then, the medium was removed, and 750 μl of acidified isopropanol was added. The optical density (OD) was measured at 540 nm using a full-wavelength microplate reader (Infinite M200, TECAN).
2.7. Cell differentiation assays
To evaluate the potential for nerve regeneration in different scaffolds, real-time PCR analysis and immunofluorescence staining were performed to quantitatively and qualitatively determine NSC differentiation. NSCs cultured on the various scaffolds for 7 days were collected, and the total RNA was isolated. Then, the cDNA was synthesized using a PrimeScript RT reagent kit (Takara Bio, Japan) according to the manufacturer's instructions. The primer sequences specific for the target gene used for qRT-PCR are listed in table 1. The qPCR amplification was performed as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 30 s, 58 °C for 1 min, and 72 °C for 1 min. The gene expression value was normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). For immunofluorescence staining, NSCs were cultured on different films in differential medium containing 1% foetal bovine serum (FBS, Life Technologies) without bFGF and EGF. After 7 days, the cells were fixed in 4% paraformaldehyde for 60 min, extracted with 0.1% TritonX-100 (Sigma) for 10 min and blocked with 10% goat serum (Sigma) for 2 h. Tuj-1 (1:200; Abcam), the primary antibodies, were incubated overnight at 4 °C, and the secondary antibodies were incubated for 2 h at room temperature, followed by staining with 4',6-diamidino-2-phenylindole (DAPI). The stained cells were observed on a confocal laser scanning microscope (LSM 780, ZEISS).
Table 1. List of genes and the primer nucleotide sequences.
| Gene | Primer sequence (5'-3') |
|---|---|
| Tuj-1 | F GATCGGAGCCAAGTTCTG; R GTCCATCGTCCCAGGTTC |
| GAPDH | F TCGCCAGCCGAGCCA; R CCTTGACGGTGCCATGGAAT |
2.8. Establishment of the spinal cord injury model
All animal procedures complied with the Guidelines for the Care and Use of Laboratory Animals and were conducted under the supervision of the Institutional Animal Care and Use Committee of Jilin University. Female SD rats (200–250 g) were purchased from the animal centre of Jilin University. Rats were randomly divided into the PLGA group (n = 8), PDA-PLGA group (n = 8), PLGA/NGF group (n = 8) and PDA-PLGA/NGF group (n = 8). Various scaffolds were chosen for implantation into the corresponding groups. The rats were anaesthetized with pentasorbital sodium (0.1%) and the hair of the back was shaved and further removed using depilatory cream. The paravertebral muscles were separated, and the exposed vertebral plates were removed using a rongeur. After exposing T9 spinal cord tissue, Venus scissors were used to cut 2-mm long section of spinal cord tissue. The scaffolds were cut into 2 × 3 × 3 mm3 cylinders, which were transplanted into the spinal cord tissue defect. Cotton swabs were used to stop bleeding. Then, the soft tissues were sutured, and the skin was closed.
2.9. Assessment of locomotor recovery
The BBB locomotor rating scale was adopted to evaluate the functional recovery of hindlimbs. Animals were assigned new identification codes after surgery to ensure that behaviour performances were rated in a blind manner. The BBB ratings follow a 21-point scale designed to assess hindlimb locomotor recovery after SCI.
2.10. Histology detection
To better observe the therapeutic effects of the scaffolds, HE staining was conducted at 4 weeks after surgery. The spinal cord from T7-T11 was retrieved and fixed in 4% (v/v) formaldehyde for 48 h. The segments were then cut into 15-mm thick sections and embedded in OCT. The tissue was then subjected to haematoxylin and eosin staining following standard protocols. The cavity area of the spinal cord was analysed by Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Cavities within 3 mm rostral or caudal to the injury/graft site were measured blindly.
2.11. Statistical analysis
All quantitative data were analysed with OriginPro 8.0 software (Origin Lab Corporation, USA) and presented as the mean ± standard deviation. Statistical differences were determined by one-way analysis of variance (ANOVA). A value of p < 0.05 was considered significant.
3. Results
3.1. Characterization
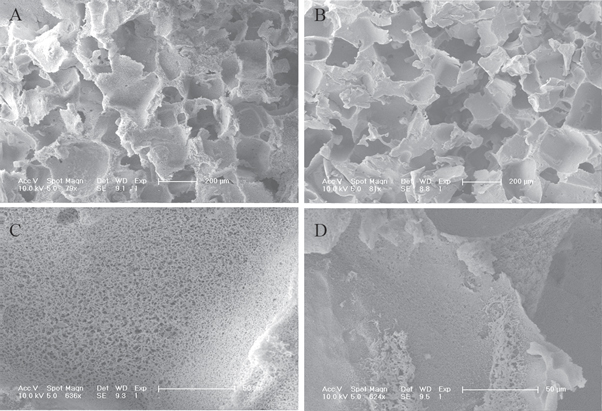
In this study, we first prepared PLGA scaffolds and PDA-PLGA scaffolds, and their surface characteristics were determined. The surface micro-morphologies of the PLGA and PDA-PLGA scaffolds were observed by electron microscopy. Pore structures with diameters between 200–400 μm were found in the scaffolds (figures 2(A), (B)). The pore surface has microchannels that connect the internal and external portions of the scaffolds, and the apertures of the microchannels decreased after the surface of the scaffolds was modified by PDA (figures 2(C), (D)). We used EDX to analyse the surface elements on each material. As shown in figure 3(A), the main elements present on the PLGA scaffolds were carbon (C) and oxygen (O). However, nitrogen (N) was observed in the EDX spectrum of the PDA-PLGA scaffolds (figure 3(B)), which confirmed that polydopamine was successfully attached to the surface of the PLGA scaffolds.
Figure 2. The microcosmic structure of untreated PLGA scaffolds and PDA-PLGA scaffolds. (A), (C): SEM images of untreated PLGA scaffolds. (B), (D): SEM images of PDA-PLGA scaffolds. Scale bar: 200 μm (C), (D) and 50 μm (E), (F).
Download figure:
Standard image High-resolution imageFigure 3. EDX analysis of PLGA (A) and PDA-PLGA (B) scaffolds.
Download figure:
Standard image High-resolution image3.2. Hydrophilicity determination
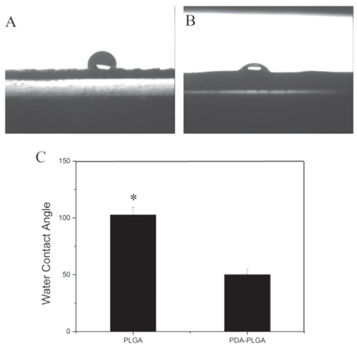
The hydrophilicity of the material is important for cell adhesion and growth. The contact angle was measured to determine hydrophilicity. As shown in figure 4, the contact angles of the PLGA group and PDA-PLGA group were (102.7 ± 6.6°) and (50.1 ± 4.9°), respectively.
Figure 4. Water contact angle of pure PLGA scaffolds (A) and PDA-PLGA scaffolds (B). (C). Average water contact angle of PLGA and PDA-PLGA scaffolds. (n = 4; *p < 0.05).
Download figure:
Standard image High-resolution image3.3. NGF binding efficiency and release curve
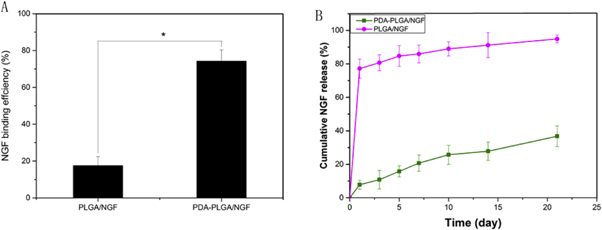
In this study, the binding efficiencies and release curves of NGF on different scaffolds were evaluated by ELISA. Figure 5(A) shows that the adhesion rates of NGF on pure PLGA and PDA-PLGA scaffolds were 17.55 ± 4.84% and 74.21 ± 6.13%, respectively. The release profile showed that approximately 8% of the NGF was released from the PDA-PLGDA scaffolds on the first day, and 37% had been released at 28 days, which indicated that NGF can undergo sustained released from PDA-PLGDA scaffolds over a long period. However, for the pure PLGA scaffolds, a burst release with 77% NGF was found on the first day, and 95% of NGF had been released at 28 days.
Figure 5. Binding efficiency of NGF onto PLGA and PDA-PLGA scaffolds (A) and cumulative release of NGF from PDA-PLGA scaffolds (B). (n = 3;*p < 0.05).
Download figure:
Standard image High-resolution image3.4. Proliferation of the NSCs
The proliferation of the NSCs on different scaffolds was observed by the MTT method at 1, 3, and 5 days. As shown in figure 6, there were no significant differences in the proliferation of NSCs among these four groups after 1 day (p > 0.05). We found that the OD values in the PLGA/NGF group and PDA-PLGA/NGF group were significantly higher than those in the PLGA group and PDA-PLGA group, respectively, at 3 days (p < 0.05). Furthermore, after NSCs were cultured for 5 days, the OD value in the PDA-PLGA group was significantly higher than that in the PLGA group, while PDA-PLGA/NGF most effectively enhanced cell proliferation among all the groups at 5 days.
Figure 6. Proliferation of NSCs cells in different porous scaffolds by MTT.
Download figure:
Standard image High-resolution image3.5. Differentiation of NSCs
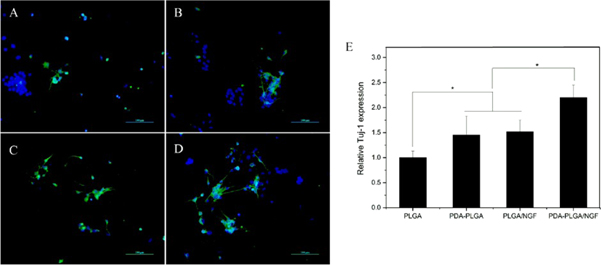
The neuronal differentiation potential of NSCs on the surface of biomaterials is closely related to the neural therapeutic effect of the implants. Therefore, the expression of Tuj-1 as a marker for neurons was quantitatively analysed using qRT-PCR. A significantly higher level of Tuj-1 gene expression was observed in the PDA-PLGA/NGF groups than in the other groups (p < 0.05, figure 7). The mRNA levels of Tuj-1 in the PLGA/NGF group and PDA-PLGA group were significantly higher than that in the PLGA group, while there was no significant difference between these two groups. To better observe neuronal cell differentiation, the expression of Tuj-1 in NSCs was evaluated by immunofluorescence staining. After 7 days of culture, the PDA-PLGA/NGF group showed the highest level of Tuj-1 expression. Moreover, neurons derived from NSCs in the PDA-PLGA/NGF group generally showed longer axons than those in the other groups.
Figure 7. Neuronal differentiation analysis of NSCs in different scaffolds. Fluorescent staining observation of the NSCs cultured on the films (A). PLGA. (B). PDA-PLGA (C). PLGA/NGF (D). PDA-PLGA/NGF) and quantitative real-time PCR analysis (E) Scale bar: 100 μm.
Download figure:
Standard image High-resolution image3.6. Animal behaviour function
To further explore the therapeutic effect of the nerve scaffold on SCI in vivo, we established a rat T9 transection SCI model. All rats were first paralysed by SCI and then displayed gradually partial recovery over several days. The BBB scores of the PLGA and PDA-PLGA groups were not significantly different. The PDA-PLGA/NGF scaffolds and PLGA/NGF scaffold implantation dramatically enhanced behaviour function compared to the PLGA group and PDA-PLGA group (p < 0.05, figure 8). Furthermore, the BBB score of the PDA-PLGA/NGF group was the highest among the four groups (p < 0.05).
Figure 8. BBB score of spinal cord injured rats.
Download figure:
Standard image High-resolution image3.7. Histological observation
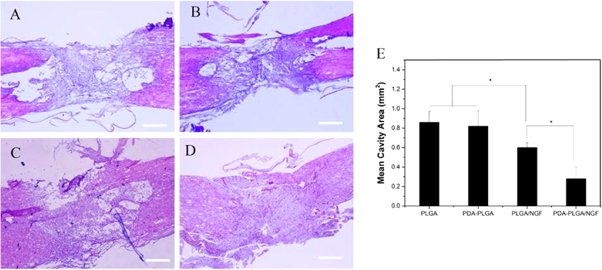
Pathological differences between the spinal cord samples from the experimental groups at 4 weeks after treatment are shown in figure 9. The neurons in the injured tissue had shrunk and were deformed. The cavity areas of the PLGA group (0.86 ± 0.11 mm2) and PDA-PLGA (0.78 ± 0.20 mm2) were significantly larger than those of the PLGA/NGF group (0.62 ± 0.08 mm2) and the PDA-PLGA/NGF group (0.28 ± 0.12 mm2). The cystic cavity forms a natural physical obstacle for further nerve fibre regeneration. Our results confirmed that PDA-PLGA/NGF administration explicitly promoted SCI recovery.
Figure 9. HE staining of spinal cord tissue. (A). PLGA. (B). PDA-PLGA. (C). PLGA/NGF. (D). PDA-PLGA/NGF (E). Mean cavity area of injury site. Scale bar: 500 μm. (n = 3;*p < 0.05).
Download figure:
Standard image High-resolution image4. Discussion
In recent years, with the rapid development of tissue engineering, different types of biological materials have been tested for SCI repair. Although SCI activates endogenous NSCs and exploits their repair mechanisms, their repair effect is far from sufficient [1, 17, 18]. Therefore, the use of biomaterials loaded with related factors can not only provide support for the injured spinal cord tissue but the bioactive factors can promote nerve regeneration.
In this experiment, we first synthesized the PLGA nerve scaffold. Electron microscopy showed that the scaffolds of each group had a pore sizes between 200 and 400 μm, which is basically consistent with the size of the porogen used. These pore structures can provide space for the nerve cells to proliferate and differentiate inside the scaffold. On the pore surface of the scaffold, microchannels with inner and outer diameters of approximately 1 μm were observed in each material. These microchannels facilitate the timely exchange of cellular metabolites and nutrients. The smaller pores in the PDA-PLGA scaffold were considered to be caused by formation of a polydopamine coating. To verify the successful modification of the PDA, EDX was used to analyse the elemental composition of the scaffold. A nitrogen (N) peak appeared in the EDX spectrum of the PDA-PLGA group, which proved that polydopamine had successfully adhered to the surface of the scaffold. The hydrophilicity of the material plays an important role in cell adhesion and growth [19, 20]. Our results indicated that PDA modification can greatly improve the hydrophilicity of the material, which compensates for the challenges associated with the hydrophobicity of the PLGA materials. Our results also establish a basis for the subsequent adhesion and growth of nerve cells on the surface of materials.
The results of ELISA showed that PDA could significantly improve the adsorption capacity of PLGA scaffolds for NGF. Moreover, ELISA showed the sustained release of NGF. The strong adsorption capacity of PDA for proteins was mainly due to specific interactions between the catechol groups and the proteins [21, 22], and the sustained release of NGF from the PDA-PLGA scaffold was extremely beneficial for the long-term and stable enrichment of NGF at the injured site.
The proliferation and neuronal differentiation ability of the NSCs is the main indicator for evaluating nerve regeneration in vitro [6, 22–24]. Our results indicate that the PDA-treated scaffold significantly promoted the proliferation and neuronal differentiation efficiency of NSCs compared to the simple PLGA scaffold. Combined with our previous study on the hydrophilicity of the material, we speculated that this may be due to the increased hydrophilicity of the PDA-PLGA scaffold enhancing the adhesion and proliferation of NSCs. When the PDA-PLGA scaffold adhered NGF, the proliferation of NSCs and the neuronal differentiation efficiency were further improved. Studies have shown that NGF can effectively promote the proliferation of NSCs in vitro while increasing the differentiation of NSCs into neurons [9, 25, 26], which is consistent with the results of this experiment. Furthermore, the axons of the neurons in the PDA-PLGA/NGF group were generally longer than those in the other groups. Considering the proven ability of NGF to promote axon growth [27], we believed that the PDA-PLGA/NGF scaffolds not only effectively promoted the proliferation and neuronal differentiation of NSCs but also played an active role in axonal elongation in the differentiated neurons.
To better evaluate the effect of treatment with the nerve scaffold in vivo, a rat spinal cord transection model was established, and the various scaffolds were implanted into the spinal injury site. The BBB score and histological results indicated that the PDA-PLGA/NGF and PLGA/NGF implants could clearly restore hindlimb motor function and reduce spinal cord tissue defects compared with the PDA-PLGA and PLGA implants. The cavity at the injury site can block nerve regeneration, which is one of the main factors causing SCI treatment failure [28]. Although we demonstrated that PDA-PLGA scaffolds and PLGA/NGF scaffolds had similar abilities to promote NSC proliferation and neuronal differentiation in vitro, the rats implanted with PLGA/NGF scaffolds showed significantly better recovery than those implanted with the PDA-PLGA scaffolds. In addition, PDA-PLGA/NGF scaffold treatment led to the best neurogenesis outcome in vitro and in vivo. Our results showed that NGF enrichment at the injured site was a key factor in nerve regeneration, while the nerve repair function of pure PDA-PLGA scaffolds for SCI was very limited.
5. Conclusion
In summary, a novel PDA-PLGA/NGF nerve scaffold was prepared, and its effects on neurogenesis in vitro and in vivo were investigated. We proved that the PDA-PLGA/NGF scaffold can significantly promote the proliferation and neuronal differentiation of NSCs in vitro. Moreover, the scaffold can effectively promote the recovery of nerve function after implantation into the rat spinal cord transection model. Therefore, the PDA-PLGA/NGF scaffold can not only maintain growth factor bioactivity using a simple manufacturing process but also show great potential for improving nerve regeneration, making it a promising nerve scaffold for subsequent clinical applications.
Acknowledgments
This study was supported by the by the National Natural Science Foundation of China, No. 81672263, No.31572217.