Abstract
Current lithium-ion batteries (LIBs) suffer from poor cycling and rate performance at low temperature (LT), which prohibit their applications in cold climate area and winter. As temperature decreases, the Li+ desolvation energy and electrode polarization increase, which lead to Li dendrite formation and battery performance fading. Herein, we report a new material surface engineering strategy to enhance Li-storage performance at LT in MXene-(Ti3C2Tx) by substituting O selectively for F termination. The pristine MXene was processed calculation at 300 °C in different atmospheres to control the partial O substitutions. The x-ray photoelectron spectroscopy (XPS), Raman spectroscopy and high-resolution transmission electron microscope (HRTEM) have been used to disclose the surface element state and fine structure. Investigated as anode of LIBs, the Ti3C2Tx(O) sample displays an improved capacity and cycle life both at −20 °C and room temperature. A discharge capacity of 226 mAh g−1 (the 10th cycle) is observed and still keep 193.9 mAh g−1 even after 1000 cycles. This is far better than that of graphite anode. Furthermore, the discharge capacity is 405 mAh g−1 (the 10th cycle) at room temperature (100 mA g−1). Even after 500 cycles, it remains 403 mAh g−1, which is much better than Ti3C2Tx(F/OH). Density functional theory (DFT) calculations show that the Li ion migration barrier could be greatly decreased with O terminal group, rationalizing the improved electrochemical performance. The O-rich surface enhance the electrolyte wettability, facilitate the Li ion desolvation and speed up the Li insertion into layered Ti3C2Tx(O). The present result demonstrates that the surface engineering strategy could promote the Li storage performance in MXene especially at LT.
Export citation and abstract BibTeX RIS
1. Introduction
Lithium-ion batteries (LIBs) are gaining great success in the field of portable energy storage device, electric vehicles (EVs) as well as smart grid [1, 2]. Recently, tremendous efforts have been devoted to improving the energy density, cycle stability and rate capability which satisfy meet the rapid development of increasing market demands. However, the performance fading at low temperature (LT) and possible safety concerns prohibit their widely applications for EVs and smart grid in cold climate and winter [3, 4]. The main reasons accounting for the poor LT battery performance includes: the sluggish solvation/desolvation of Li+ and Li+ intercalation/de-intercalation reactions kinetics, insufficient ionic conductivity, as well as severe electrode polarization [5, 6]. To solve these aforementioned challenges, various approaches have been explored [4–9]. Improved cycle stability and capacity are obtained by investigating new electrolyte additives and liquefied gas based electrolytes [6]. The study of organic materials and prelithiated hard carbon as well as 3D porous CuZn alloy have been proved to be effective in enhancing the Li+ diffusion ability at LT [10]. However, it still hardly meets the requirements of large-scale commercialization. It is urgent to develop novel electrode materials and find the corresponding mechanism which is suitable for anode of LIBs at LT.
MXene, a new kind of 2D layered transition metal carbides, has received widespread attention since it was obtained successfully from MAX phases [11–17]. The general formula is Mn+1XnTx (n = 1–3), in which M represents early transition metals (Ti, V, Cr, Nb etc), X is C or N and T is the surface terminations [13, 18–22]. Due to its good electrical and ionic conductivity, it has been widely studied as electrodes for Li/Na ion batteries [23–25] and supercapacitors [26–29]. The Li ions penetrate and storage inner MXene sheets layers with a theoretical capacity of 447.8 mAh g−1 [30]. However, the −F termination group usually deteriorate the initial discharge capacity and cycle stability due to the undesirable electrode/electrolyte interface. Very recently, Kajiyama et al report steric chloride termination of Ti2CTx to improve Li-ion accessibility through open interlayer space [31]. Ahmed et al introduce O terminated group MXene to enhance the Li-storage performance as anode for LIBs [32]. The progress demonstrates that the optimization of termination group could greatly influence the electrode/electrolyte interfacial contact and thus improve the battery performance. However, it is still blind to us what is the mechanism of surface engineering in promoting the Li ions insertion into MXene layers. In addition, it is still blank whether this property depends on operating temperature.
Herein, we report a material surface engineering strategy to enhance the LT Li-storage performance in MXene-(Ti3C2Tx). The as-prepared pristine MXene was processed at 300 °C in different atmospheres to regulate the surface O termination group. To characterize modified surface and structures, the samples were subjected to x-ray photoelectron spectroscopy (XPS), Raman spectroscopy and high-resolution transmission electron microscope (HRTEM). The Li storage performance of Ti3C2Tx(O) have been studied both at RT and LT (−20 °C). To deeply investigate the electrochemical improvement mechanism after partial O substitution, we performed density functional theory (DFT) calculations to evaluate the Li diffusion barrier in MXene with different terminal groups. We also tried to rationalize the relationship between optimized termination O group and its temperature-dependent Li-storage performance.
2. Experimental section
2.1. Synthesis materials
MAX phase Ti3AlC2 was prepared by ball-milling a mixture of Ti2AlC powder (>90 wt%, Forsmon) and TiC powder (99%, Johnson Matthey Electronic, NY) with a molar ratio of 1:0.8 for 18 h. The mixture was sintered at 1350 °C for 2.5 h under Ar. Then the material was ball milled for 6 h to smash the bulk particles.
To synthesize Ti3C2Tx(F/OH) MXene nanosheet, Ti3AlC2 MAX phase was treated with 40% concentrated HF (American Chemical Society grade, BDH) for 6 h at 30 °C to etching the Al out. The as-obtained powder was then washed by using deionized water for several times until the pH > 6.5, followed by drying in a vacuum oven at 60 °C for 12 h. The MXene-Ti3C2Tx(O) with more O-terminated surfaces was obtained by annealing Ti3C2Tx(F/OH) powder at 300 °C under ambient air. And the Ti3C2Ox/TiO2 were obtained by annealing Ti3C2Tx(F/OH) powder in pure O2 at 400 °C for 1h.
2.2. Characterizations
Powder x-ray diffraction (XRD) data were collected on a Miniflex 600 by employing a Cu Ka (λ = 0.154 18 nm) radiation source. The morphology was observed by scanning electron microscopy (SEM, Verios 460L Field Electron and Ion Co.) and HRTEM with field-emission gun (HRTEM, Talos F200 X, FEI). The surface elemental information was measured by x-ray photoelectron spectrometer (Escalab 250Xi, ThermoFisher Scientific) using monochromatized Al Kα x-rays as the excitation source. Raman spectrum were measured with a Horiba Scientific at excitation laser wavelength of 532 nm.
2.3. Electrochemical measurements
Electrochemical performance was carried out in CR2032 coin type cells. The working electrodes were prepared by coating the slurry of the active materials, Ketjen black, and sodium carboxymethyl cellulose at a weight ratio of 7:2:1 onto copper foil and dried in a vacuum at 60 °C. The electrolyte was composed of 1.0 M LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DEC) with a volume ratio of 1:1. Celgard film was employed as the separator. The coin cells were assembled in an argon-filled glove box (Mikrouna) with the concentrations of O2 and H2O < 0.1 ppm.
The galvanostatic discharge–charge measurements were performed in the potential range of 0.01–3.0 V versus Li+/Li on a Land battery test system (Land CT2001A). And the temperature was controlled by a high and LT environmental test oven. Cyclic voltammetry (CV) and impedance data were collected by using an electrochemical workstation (CHI 760E).
2.4. Theoretical calculations
DFT based first-principles calculations were performed using the Vienna ab initio simulation package (VASP) [33, 34] within the projector augmented-wave approach [35]. Generalized gradient approximation (GGA) in the parameterization of Perdew, Burke, and Ernzerhof (PBE) [36] pseudopotential was used to describe the exchange–correlation potential. The Li-ion diffusion barrier was evaluated with the nudged elastic band (NEB) method [37]. This approach duplicates a series of images between the starting point and the end point of diffusing ion to simulate the intermediate states, with the positions of the starting point and the end point fixed. The supercell comprises 4 × 4 × 1 conventional unit cells, corresponding to 32 formula units of Ti3C2O2/Ti3C2O2. For the large supercell adopted in NEB calculations, only the Γ point is adopted for k-point sampling to reduce the computational cost. The plane-wave cut off was set to 520 eV. Geometry optimizations were performed by using a conjugate gradient minimization until all the forces acting on ions were less than 0.01 eV Å−1 per atom.
3. Results and discussion
3.1. Material characterization
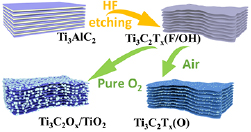
The preparation and surface engineering of MXene Ti3C2Tx is schematically shown in figure 1. Firstly, MXene Ti3C2Tx(F/OH) is obtained through etching Al out by HF from Ti3AlC2. The –F or –OH group is formed as terminals lying on the aqueous solution condition. Exposed to different atmospheres at 300 °C, the O replace F on the surface of MXene gradually. The partial O substituted Ti3C2Tx(O) and totally oxidized Ti3C2Ox/TiO2 can be obtained in ambient air and pure O2, respectively.
Figure 1. Schematic illustration of the fabrication process of pristine MXene-Ti3C2Tx(F/OH), more O functional group in MXene-Ti3C2Tx(O) and Ti3C2Ox/TiO2.
Download figure:
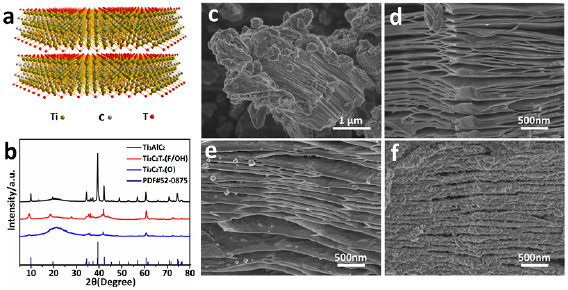
Standard image High-resolution imageThe characterizations of as-prepared materials are shown in figure 2. We can understand its structural characteristics more intuitively as displayed in figure 2(a). The 2D sheets are obtained by means of selectively etching Ti3AlC2 [11, 14]. The presence of phases and structures has been identified by XRD experiments as illustrated in figure 2(b). The pattern of Ti3AlC2 is consistent with the standard card (JCPDS card No. 52-0875) basically, in which a small amount of TiC served as impurities. As for the Ti3C2Tx(F/OH), the (0 0 2) peak shifts from 2θ = 9.5° to 2θ = 8.7° and the corresponding c axis lattice parameter of ex-Ti3C2 changes from 18.5 to 20.3 Å [21, 38]. The layer distance increased as the Al etching out. The crystallinity decreased after O partially substituting while the layered MXene structures still maintains. After deeply oxidation in pure O2, the main phase can be indexed as TiO2 (JCPDS card No. 21-1272) as displayed in figure S1 (stacks.iop.org/TDM/6/045025/mmedia).
Figure 2. Structural characterization of the samples. (a) Crystal structure of Ti3C2Tx. (b) XRD patterns of the different samples. SEM images of pristine Ti3AlC2 (c), as-fabricated Ti3C2Tx(F/OH) (d), Ti3C2Tx(O) (e) and Ti3C2Ox/TiO2 (f).
Download figure:
Standard image High-resolution imageFigure S2 shows the Raman spectra of the Ti3C2Tx(F/OH), Ti3C2Tx(O), and Ti3C2Ox/TiO2 samples. Compared to the Ti3C2Tx(F/OH) sample, Ti3C2Tx(O) sample shows a major peak centered at 154 cm−1 corresponding to anatase TiO2 and the intensity of which has slight changed with Ti3C2Tx(F/OH) sample. This proved that a small amount of TiO2 appears on the surface of the material [32, 39]. The other three broad Raman peaks centered nearly around 254, 410, and 610 cm−1 representing the vibration modes can be assigned to nonstoichiometric titanium carbide [40]. Compared to the Ti3C2Tx(F/OH) sample, the band centered around 610 cm−1 shifts to higher energy for all annealed samples.
It has been reported that for layered materials, as the layer thickness decreases, the band position shifts to higher energy representing a slight hardening of the bonds as the layer thickness decreases [39, 41]. Raman spectra indicate a slight thinning of layers in the annealed samples, leading to larger interplanar distance.
SEM was carried out to study the morphology evolution. As shown in figures 2(c) and (d), an accordion-like multilayer structured MXene Ti3C2Tx(F/OH) is observed after etching out Al from Ti3AlC2, which confirms successful exfoliation of the pristine Ti3AlC2. Regular nanoparticles formed on the sheet surface after heat treatment in the air (figure 2(e)). And the sheets are completely covered by nanoparticles with a size of 20–30 nm after deeply oxidation as revealed in figure 2(f). The nanoparticle is confirmed to be TiO2 combined with the XRD results. What's more, it still maintains layered structure, which is consistent with the results of SEM.
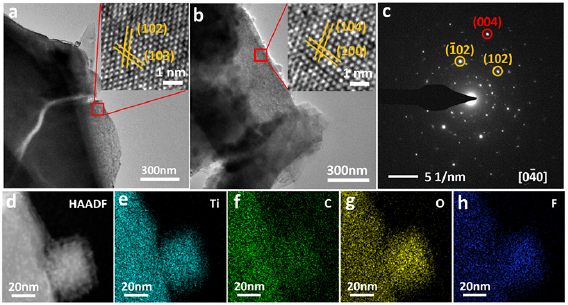
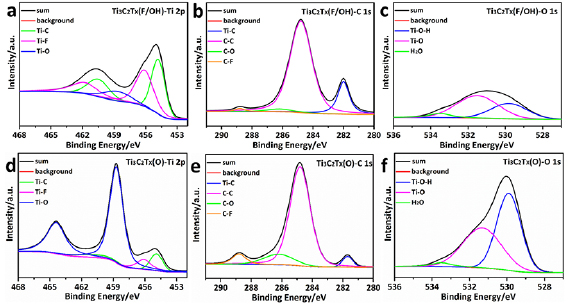
To further understanding the structural evolution after heat processing, transmission electron microscope (TEM) and the HRTEM was carried out as shown in figure 3. Figure 3(a) and inset display the TEM and HRTEM images of Ti3C2Tx(F/OH), after etching process, which displays clear adjacent lattice fringes with d-spacings of 0.255 and 0.244 nm, corresponding to the (1 0 2) and (1 0 3) plane of Ti3C2 respectively [42]. Figure 3(b) and inset display the structural information of Ti3C2Tx(O). The fringe spacing measured to be 0.265 and 0.223 nm, which can be indexed to the (1 0 0) and (1 0 4) planes of Ti3C2, respectively, the crystallinity of the basal planes of the Ti3AlC2 phase is preserved well. Therefore, it can be reasonably concluded that the MAX crystal structure (JCPDS card no. 52-0875) of the basal planes is maintained in the MXene. Selected area diffraction (SAED) pattern of the Ti3C2Tx(F/OH) sheets (figure S3(a)) demonstrates that the MXene sheets retain the hexagonal symmetry and crystallinity of the basal planes of the parent Ti3AlC2 phase. After heat processing, the SAED of Ti3C2Tx(O) (figure 3(c)) shows some reflecting peak corresponding to TiO2, which was produced by the local heat developed at surface modification anneal processing of the Ti3C2Tx(F/OH). Furthermore, figure 3(d) displays the dark-field TEM image of the Ti3C2Tx(O) and the corresponding Energy Dispersive Spectroscopy (EDS) maps of the Ti3C2Tx(O) in figures 3(e)–(h) show a uniform distribution of Ti, C, F, and O. The dark-field TEM image and EDS maps of the Ti3C2Tx(F/OH) are shown in figure S3, which also indicate the uniform distribution of elements. The surface elemental analysis was performed on XPS. The spectrum demonstrated in figure 4 clearly reveals that Ti, C and O are presented on the surface of Ti3C2Tx(F/OH) and Ti3C2Tx(O), respectively. The Ti 2p core level is fitted with three doublets (Ti 2p3/2 − Ti 2p1/2) with a fixed area ratio equaling to 2:1 and the energy difference between Ti 2p3/2 − Ti 2p1/2 peaks is 5.7 eV [26, 43]. As displayed in figure 4(a) of the high-resolution Ti 2p spectrum, the peaks at 461.8eV, 460.1.0 eV, 458.7 eV, 456.1 eV, and 454.8 eV can be assigned to Ti–O, Ti–F and Ti–C of Ti3C2Tx(F/OH), respectively. It indicates that a small amount of titanium was oxidized during the etching process in HF aqueous solution. Figure 4(b) show the C 1s spectrum which means the peaks at 282.0, 284.8, 286.2 and 288.8 eV corresponds to Ti–C, C–C, C–O, and C–F, respectively [43, 44]. The peaks for O 1s locate at 529.8, 531.5 and 533.5 eV are associated with Ti–O–H, Ti–O and H2O respectively [45, 46]. Figures 4(e) and (f) show the XPS spectra of Ti3C2Tx(O) which is processed in ambient air at 300 °C. The intensity of Ti 2p3/2 peaks at 456.1 and 454.8 eV decreases significantly, accompanied with an increase of Ti–O peak centered at 458.7eV, compared with those of the Ti3C2Tx(F/OH). This indicates that Ti on the surface has been oxidized after processing. Correspondingly, for the spectrum of C 1s (figure 4(e)), the intensity of Ti–C centered at 281.7 eV decreases substantially. Meanwhile, the intensity of C–O centered at 286.2 eV increases, and the intensity of C–C centered at 284.8 eV remains unchanged. As the high-resolution XPS spectra of O 1s, compared figure 4(c) with 4(f), we can come to realize that the concentration of O on the surface has been greatly improved. These results indicate that the content of O as termination group are increased on the surfaces.
Figure 3. (a) and (b) The TEM and HRTEM image of the Ti3C2Tx(F/OH) and the Ti3C2Tx(O). (c) The SAED pattern of Ti3C2Tx(O). (d) Dark-field TEM image of the Ti3C2Tx(O). (e)–(h) The corresponding elemental mapping of the Ti, C, O and F.
Download figure:
Standard image High-resolution imageFigure 4. High-resolution Ti 2p, C 1s and O 1s XPS spectra. XPS results of (a)–(c) for the untreated Ti3C2Tx(F/OH). (d)–(f) For the Ti3C2Tx(O).
Download figure:
Standard image High-resolution image3.2. Electrochemical characterization
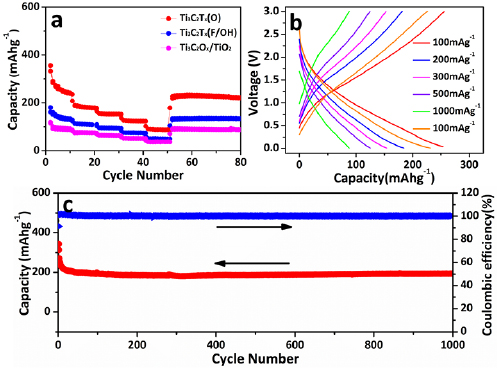
To further explore the surface engineering effects on Li-storage performance at varied temperatures, the electrochemical performance was investigated carefully by using CR2032 coin cells with lithium metal anode at both room temperature and −20 °C. Figure 5(a) displays the rate performance of Ti3C2Tx(F/OH) and Ti3C2Tx(O) at −20 °C. With the increasing of current densities from 100 to 1000 mA g−1, the discharge capacities decreased correspondingly in which demonstrate a order of Ti3C2Tx(O) > Ti3C2Tx(F/OH) > Ti3C2Ox/TiO2. Even at a current density of 1000 mAg−1, Ti3C2Tx(O) displays a discharge capacity of 88.4 mAh g−1, far better than 51.2 mAh g−1 for Ti3C2Tx(F/OH) and 36.6 mAh g−1 for Ti3C2Ox/TiO2. When returned to a current density of 100 mAh g−1, the discharge capacity also recovered perfectly. This indicates the good cycle stability with prolonged cycles.
Figure 5. Electrochemical characterization at −20 °C. (a) Rate performance of different materials. (b) Charge–discharge profiles at different current density. (c) Cycling profiles at 100 mA g−1 for 1000 cycles at −20 °C.
Download figure:
Standard image High-resolution imageThe commercial graphite was also adopted as anode for the comparing the Li-storage performance at LT. As shown in figure S5, the graphite only shows capacity of 77 mAh g−1 (100 mA g−1) and 15.4 mAh g−1 (1000 mA g−1), which are much lower than that of Ti3C2Tx(O). The voltage profiles of Ti3C2Tx(O) are shown in figure 5(b). Similar with previous reported MXene-based electrode materials, the present Ti3C2Tx(O) electrode displays a capacitor-like behavior upon Li ions insertion/desertion process [27, 29]. Figure 5(c) shows the long cycle performance of up to 1000 cycles at a current density of 100 mA g−1 at −20 °C. The capacity retention at 1000th cycle is 85.8% (compared with 10th cycle) and corresponding to a capacity lost of 0.014% per cycle, We also carefully compared the current performance with previously reported low-temperature LIBs and a table was given at table S1. It could be found that this work is among one of the best previous results. It is generally considered that there are several reasons account for the performance fading with the decreasing operating temperature. The increased Li ions desolvation energy and electrode polarization at LT directly deteriorate the amounts of Li ions which can inserted into electrode materials [3, 5, 8]. The present enhance of Li ion on storage performance in partial oxidized Ti3C2Tx(O) is associated with the well electrode/electrolyte contact and improved Li ions desolvation capability even at LT.
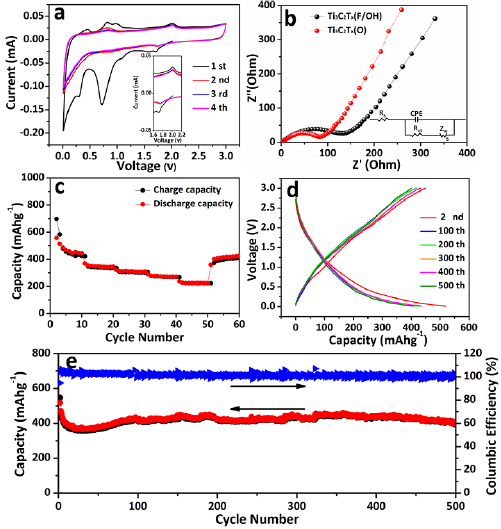
The electrochemical properties of Ti3C2Tx(O) at room temperature were further investigated and shown in figure 6. The CV shown in figure 6(a) were carried out ranging from 0.01 to 3 V (versus Li+/Li) at a scan rate of 0.1 mV s−1. In the initial negative scan, four distinct main reduction peaks can be observed at 1.72, 1.09, 0.73 and 0.30 V, respectively. There are two oxidation peaks at 0.83 and 2.0 V appeared at the following positive scan. The irreversible peaks mainly due to the formation of solid electrolyte interphase (SEI) and some unknown side reactions [47, 48]. The enlarged curves inset figure 6(a) exhibit good reprodu-cibility and the oxidation peaks almost overlap, indicating high reversibility. The CV curves of the pristine Ti3C2Tx(F/OH) are presented in figure S6. There are only three distinct main reduction peaks observed at 1.63, 0.75 and 0.36 V in the first negative scan process. The shoulder peak at 1.09 V disappeared may be associated with the good Li ions absorption capacity on the surface of Ti3C2Tx(O). It is one of the possible reasons for the improved rate performance of partial surface oxidized Ti3C2Tx(O). Electrochemical impedance spectra (EIS) were carried out to explore the resistant evolution. Figure 6(b) show the Nyquist plots of the Ti3C2Tx(O) and Ti3C2Tx(F/OH) electrodes after 50 cycles, the Nyquist plots measured for Ti3C2Tx(F/OH) and Ti3C2Tx(O) electrodes at different cycle number displayed in figure S7. Insertion figure showed the equivalent circuit model consists of Rs: element accounts for the total ohmic resistance of electrolyte, CPE: the constant phase element that simulates nonideal behavior of the capacitor by combining the surface modification capacitance and the double layer capacitance as a series, Rct: resistance to charge transfer on the electrode and Zw: the Warburg impedance of Li+ solid phase diffusion. And the resulting Rct of electrodes after different cycles were concluded in table S2. Disclosing that surface-modified MXene can boost the charge transfer process greatly and thereby resulting in better rate performance.
Figure 6. Electrochemical characterization at room temperature. (a) CV curves at scan rate of 0.1 mV s−1of Ti3C2Tx(O) (inset: partial enlargement). (b) EIS of the Ti3C2Tx(O) and Ti3C2Tx(F/OH) electrodes after 50 cycles. (c) Rate performance of the Ti3C2Tx(O). (d) Charge–discharge curves at current density of 100 mA g−1. (e) Cycling profiles at 100 mA g−1for 500 cycles.
Download figure:
Standard image High-resolution imageThe rate performance of the Ti3C2Tx(O) at room temperature is show in figure 6(c). Extraordinary reversible capacities were achieved as 456 (5th cycle, 100 mA g−1), 340 (15th cycle, 200 mA g−1), 310 (25th cycle, 300 mA g−1), and 273 (35th cycle, 500 mA g−1) to 223 mAh g−1 (45th cycle, 1000 mA g−1) at room temperature. Remarkably, when the current density returned to 100 mA g−1, the capacity back to 428 mAh g−1 after 65th cycles, maintaining more than 95% of the initial value. Figure 6(d) presents the voltage profiles at selected cycles at a current density of 100 mA g−1. As can be seen in the profiles, the plateaus on the discharge-charge curves are greatly consistent with the respective peaks on the CV curves. Figure 6(e) shows the cycle performance of Ti3C2Tx(O) anode at a current density of 100 mA g−1. Even after 500 cycles, it still delivers a capacity of 404 mAhg−1 and demonstrates ultra-stable cycle performance. The result discloses that the material structure remains stable after long term cycles. Compared to the performance of Ti3C2Tx(F/OH) anode in figure S8 and Ti3C2Ox/TiO2 anode in figure S9, the Ti3C2Tx(O) anode still exhibits the highest capacity of 404 mAh g−1 and cycle stability. The gradual increase of capacity in Ti3C2Ox/TiO2 anode mainly is associated with the pulverization of TiO2 and successive decomposition of electrolyte during repeated cycles [49]. We also carefully compared the present performance with previous reported MXene-based anodes and the results are shown in table 1. It could be found that this work is among one of the best.
Table 1. Comparison of the specific capacities and cyclic properties of MXene as anodes for LIBs.
| Electrode materials | Current density (mA g−1) | Capacity (mAh g−1)/Cycle number | Refs |
|---|---|---|---|
| Mo2TiC2Tx | 1C | 143.5/160th | [13] |
| Ti3C2Tx | 260 | 410/120th | [21] |
| in-Ti3C2 | 260 | 118/75th | [25] |
| Nb2CTx | 1C | 170/100 | [47] |
| V2CTx | 1C | 260/150th | [47] |
| Ti2CTx | 1C | 110/80th | [50] |
| V2CTx | 370 | 260/20th | [51] |
| Nb4C3Tx | 100 | 380/100th | [52] |
| Ti3C2 | 260 | 155/250th | [53] |
| Ti3C2/CNF | 320 | 320/— | [54] |
| Hf3C2Tx | 200 | 146/50th | [55] |
| Ti3C2Tx/CMK-5 | 1C | 342/100th | [56] |
| Ti3C2Tx(O) | 100 | 422/100th | This work |
| 421/200th | |||
| 404/500th |
To understand the electrochemical improvement mechanism after partial O substitution, we employed DFT calculations to evaluate the Li diffusion barrier in MXene with different terminal groups. As shown in figure S10, the Li ion migration barrier has been greatly decreased with O terminal group compared with that of F terminal group. A low migration barrier would facilitate the rapid diffusion of Li ions, which may explain the improved electrochemical performance of the Ti3C2Tx(O) electrode in this work.
4. Conclusions
In summary, we investigated the surface oxygen termination in MXene based materials and their effects on Li-storage performance at both LT and room temperature systematically. The degree of surface oxygen substitution has been controlled through optimizing the sintering conditions. In ambient air, the surface O-rich Ti3C2Tx(O) could be obtained while Ti3C2Ox/TiO2 could be obtained in pure O2. Both of the surface modified samples maintain the layered structure well. Interestingly, Ti3C2Tx(O) with O-rich surface demonstrate good electrolyte wettability which promote the Li ion desolvation and facilitate the Li insertion into layers. Both at −20 °C and room temperature, Ti3C2Tx(O) demonstrates an improved Li-storage performance. A discharge capacity of 226 mAh g−1 (the 10th cycle) is observed and can still keep 193.9 mAh g−1 even after 1000 cycles at −20 °C. It is one of the best Li-storage's performance at LT among the reported anode materials. This is also far better than that of commercialized graphite anode. At room temperature, Ti3C2Tx(O) has excellent electrochemical performance, the discharge capacity is 405 mAh g−1 (10th cycle at 100 mA g−1), even after 500 cycles, it still remains 404 mAh g−1 and almost no capacity fading. As far as we know, this is the best performance among the reported MXene-based anode materials. The influence of surface end group change on the improved electrochemical performance has been explored using DFT calculations, which suggest that the Li ion migration barrier could be greatly decreased with O terminal group. The synergistic effects of increased oxygen and decreased F terminations effectively enhancing the Li+ diffusion kinetics and structure stability of the electrode, allow us to gain improved Li storage performance at varied temperatures. The present work indicates that surface engineering strategy provide a new possibility for designing and fabricating safe and high performance of anode materials for LIB suitable for cold climate areas.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21603162, U1804255), the National Science Fund for Distinguished Young Scholars (51825102), the Tianjin Municipal Science and Technology Commission (15JCYBJC52700, 17JCYBJC21500) and National Key Research Program of China 2017YFB0102000.