Abstract
Time-resolved fluorescence detection for robust sensing of biomolecular interactions is developed by implementing time-correlated single photon counting in high-throughput conditions. Droplet microfluidics is used as a promising platform for the very fast handling of low-volume samples. We illustrate the potential of this very sensitive and cost-effective technology in the context of an enzymatic activity assay based on fluorescently-labeled biomolecules. Fluorescence lifetime detection by time-correlated single photon counting is shown to enable reliable discrimination between positive and negative control samples at a throughput as high as several hundred samples per second.
Export citation and abstract BibTeX RIS
1. Introduction
Fluorescence detection is a widespread technique used in life sciences and pharmaceutical industry for e.g. wide field microscopy [1–3], flow cytometry [4, 5] cell sorting [6, 7] or high-throughput screening (HTS) [8–10]. Multiple variants of fluorescence-based detection techniques are used for sensing local physico-chemical environment properties or interactions between fluorescently-labeled biomolecules. They include fluorescence intensity (FLINT), fluorescence polarization/anisotropy (FP), single-molecule detection methods or fluorescence lifetime (FLT). Of particular interest, FLT is an intrinsic reporter of the fluorescence quantum yield of a fluorophore, and therefore of the fluorophore interactions with its microenvironment. This means in particular that parameters such as fluorophore concentration, excitation light intensity, excitation light screening or fluorescence reabsorption, which do not modify the fluorophore quantum yield, will not alter the fluorophore FLT. Instead they do alter all the other time-integrated fluorescence techniques (e.g. FLINT of FP), which makes them less reliable for the accurate sensing of fluorophore interactions. This well-known advantage of FLT detection is for instance at the origin of the very successful development of fluorescence lifetime imaging microscopy (FLIM) [11–14].
In the context of HTS, the same argument has motivated the development of innovative biochemical constructs taking advantage of FLT detection for the efficient sensing of molecular interactions [15–24]. However, although the use of FLT for HTS was first explored more than 15 years ago [25–27], it has still not been commonly applied because implementing FLT in high-throughput conditions remains technologically more demanding than intensity-based fluorescence techniques. FLT sensing has long been applied to flow cytometry [28–30] using rapid, frequency-domain, FLT detection techniques which are applicable to high-throughput conditions. Conversely, FLT detection in the time domain by time-correlated single photon counting (TCSPC) is well-known to be the most sensitive and accurate technique also offering the best FLT time resolution [31]. TCSPC may therefore appear to be the preferred approach for FLT-based biosensing, however it requires counting and time-stamping photons one by one, with at maximum one photon detection event per excitation pulse [32]. A fluorescence decay kinetic trace is thus obtained by reconstructing the histogram of the distribution of detection times, after a large number of excitation laser pulses. The method may thus appear relatively slow and was claimed to be inapplicable to high-throughput FLT detection [33]. Alternative time-domain FLT detection techniques have been specifically developed for HTS [33] or flow cytometry [34], but offering reduced sensitivity and time resolution compared to TCSPC. For instance, the so-called Direct Waveform Recording (DWR) technique [33] relies on the analog recording of the fluorescence decay kinetics triggered by single, nanosecond, intense laser shots at a multi-kHz repetition rate. This approach is much less sensitive in essence and its implementation more expensive than TCSPC because it requires much higher average laser power for excitation (i.e. 10 mW typically). Still, it was already very efficient at enabling FLT detection for HTS [20–23] and demonstrating the significant advantage of FLT [23, 35] (i.e. intrinsic reporter of local fluorophore interactions) over FLINT in this context.
In contrast to the initial motivations for developing e.g. DWR, however, several applications of TCSPC were recently demonstrated for accurate FLT detection under high throughput conditions [36–38]. In particular, using a microfluidic flow cytometer designed for efficient FLT detection by TCSPC at a throughput up to 3000 particles per minute, Nedbal et al [37] reported a comprehensive series of experiments demonstrating the superiority of FLT over FLINT for biomolecular interaction sensing in individual cells, based on the detection of Förster resonant energy transfer between ns-lived chromophores including fluorescent proteins. We instead have implemented TCSPC in microfluidic droplets and investigated the fundamental limitations imposed by the single photon detection technique on the photon acquisition rate and on the achievable signal-to-noise ratio in high throughput conditions [36]. We demonstrated that accurate discrimination between distinct FLTs (i.e. positive and negative control samples in a HTS protocol) is achievable with TCSPC at a droplet throughput exceeding 1 kHz. Droplet microfluidics (DmF) is a promising tool for biotechnologies because it lowers the volume of individual samples from μl range per well in microplates down to the 100 pl range or less in micro-droplets and it considerably increases the samples (droplets) manipulation rate [6, 39–42]. Consequently, DmF provides particularly challenging conditions in terms of throughput and required sensitivity, to assess the performances of TCSPC for high-throughput FLT detection.
In the present paper we review our latest developments towards demonstrating a high throughput, cost-efficient TCSPC detection technique. After describing the technical implementation, we in particular illustrate the potential of the approach by demonstrating the feasibility of an enzymatic activity assay relying on the detection of the FLT by TCSPC, in very high-throughput conditions, in microfluidic droplets. The choice of an enzymatic activity assay as a validation for biomolecular interaction sensing was driven by the high biotechnological interest on enzymes [43–46]. Beyond, this paradigmatic assay paves the way for the application of TCSPC to high-throughput biomolecular interactions and biotechnologies in general.
2. Materials and methods
2.1. Microfluidics
In this work water-in-oil microdroplets are used as small-volume reservoirs that contain the samples of interest and can be manipulated at very high throughput in microfluidic channels. As previously reported [36], we use the conventional 'rapid prototyping' technique [47] to manufacture polydimethylsiloxane microfluidic chips. A so-called T-junction is used for droplet generation: three aqueous inlets merge into one channel which intersects orthogonally a fourth channel flowed with immiscible oil (10:1 mixture of perfluorodecalin with 1H,1H,2H,2H-perfluorodecyltrichlorosilane). The mixing of the three aqueous solutions is initiated by the droplet formation and is further accelerated by chaotic advection induced within the droplets by the wiggling shape of the channel after the T-junction [48]. The velocity, size and concentration of the droplets are precisely controlled by regulating the flow rates in the aqueous and oil channels using a commercial flow control system (Elveflow, Elvesys, France). Droplets of less than 200 pl volume and 1 μM fluorophore or substrate concentration were used in the present work. This represents no more than 200 attomol (10−18 mol) of solute per droplet, i.e. per sample.
2.2. Enzyme activity assay
To illustrate the efficiency of the proposed FLT detection technology for HTS, we demonstrate the feasibility of an enzymatic activity assay relying on FLT detection. The assay [15, 18, 19] is designed to evidence the activity of the protease human pancreatic trypsin (from autolyzed human pancreas, Elastin Product Company, Inc., Owensville, MO, USA, catalog number TR127) on a peptide substrate tagged with the fluorescent probe PT14. Due to the structural flexibility of the peptide the probe undergoes dynamic quenching resulting in a FLT of 7 ns. When the enzyme cleaves the substrate, the probe is released, and its FLT of 14 ns is restored. Figure 1 displays the kinetics of FLT recovery after mixing 1 μM substrate with 1 or 10 nM enzyme in PBS. In this work, the fluorescently-labeled substrate was Biosyntan 9385.1 C(PT14)-GFKY-NH2, purchased from Biosyntan (Berlin, Germany). It was, delivered as dry powder, dissolved in DMSO and kept at −20 °C. For all experiments it was dissolved in PBS (phosphate buffered saline, pH = 7.4) and 0.02% v/v TWEEN (polyethylene glycol sorbitan monolaurate) was added. In the microfluidic droplets, the concentrations of substrate and enzyme were adjusted to 1 μM and 1 nM, respectively.
Figure 1. Kinetics of the PT14 FLT recovery upon substrate cleavage in the presence of 1 or 10 nM protease human pancreatic trypsin. Data recorded with a Tecan Ultra Evolution, I20044 microplate reader.
Download figure:
Standard image High-resolution image3. Results
3.1. FLT detection set-up
Figure 2 displays a sketch of the experimental set-up. A home-made fluorescence microscope is assembled to detect the fluorescence of water-in-oil droplets circulating in the microfluidic chip. A dichroic mirror (Semrock, BLP01-442R) is used to reflect the excitation light and transmit the fluorescence emission. A microscope objective (Nikon, x50, NA = 0.6) is used to focus the excitation beam to a ∼5 μm spot inside the droplets circulating in the main microfluidic channel, and to collect and collimate the fluorescence emitted by the droplets. A second objective (Nikon, x20, NA = 0.4) is used to focus the fluorescence light on a single photon avalanche diode (SPAD, ID100, Id-quantique, Switzerland). The excitation source is a diode laser (DL-4146-101S, Roithner lasertechnik, Austria) emitting at 405 nm and operated in a pulsed mode, by a home-made, cost-effective driver consisting of a current pulse generator described previously [49]. The laser pulse repetition rate is fixed at 20 MHz with 80 ps duration FWHM. The laser average power is 100 μW, attenuated to 50 μW in the microfluidic experiments performed with the PT14 probe, which is however characterized by a relatively low extinction coefficient (εmax ∼ 7600 M−1 cm−1 at 394 nm) [15].
Figure 2. Scheme of the experimental setup. A home-made fluorescence microscope is used to excite and collect fluorescence emission in microfluidic droplets (O1, O2 = microscope objectives, DM = dichroic mirror). Water-in-oil droplets are generated in a PDMS microfluidic chip at a so-called T-junction where 3 aqueous channels (C1–C3) intersect one oil channel. A low-cost TCSPC detection set-up operating at high throughput has been developed, including a cheap diode laser and its home-made driver for pulsed emission (at a 20 MHz repetition rate, 100 μW average power), a commercial SPAD and a home-made FPGA-based TDC which enables rapid, on-the-fly processing of single photon detection events.
Download figure:
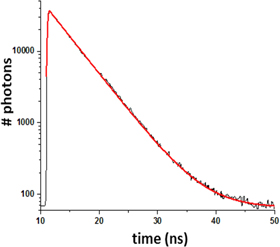
Standard image High-resolution imageAccording to conventional TCSPC experiments, a TDC is used to measure the time laps between each single photon detection event delivered by the SPAD and the previous laser pulse. As a TDC, we use here a Field-Programmable Gate Array (FPGA, Altera® Cyclone EP4CE55) board configured to realize the function of a TDC. The FPGA board actually generates the 20 MHz clock signal that triggers the laser diode driver, acquires the signal delivered by the SPAD, and measures the time delay between trigger and SPAD signals as a conventional TDC. The design of this home-made, cost-effective, accurate FPGA-based TDC was described in detail elsewhere [50, 51]. It is characterized by a 42 ps time resolution, 1280 ns full scale, and 20 ns dead time. As an illustration, figure 3 displays the fluorescence decay of fluorescein dissolved to 10 μM in PBS, acquired in a conventional spectroscopy cuvette with this FPGA-based TDC, and showing the expected monoexponential decay kinetics with a FLT of 4.2 ns.
Figure 3. Testing the home-made FPGA-based TCSPC acquisition system. Monoexponential fluorescence decay (semilog plot) of fluorescein in PBS solution measured, with the FPGA-based TDC, in a conventional 1 mm thick quartz cuvette standing in place of the microfluidic chip in the experimental set-up described above.
Download figure:
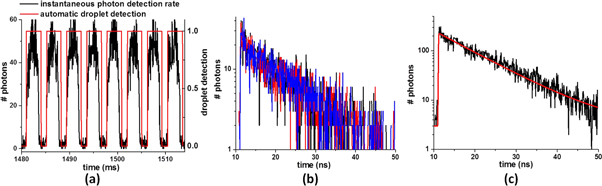
Standard image High-resolution imageThe above FPGA-based TDC is used for TCSPC FLT detection in microfluidic droplets as illustrated in figure 2. A typical experimental run consists of circulating droplets of given size at a controlled flow rate and concentration, while detecting and time-stamping single photons continuously over seconds to minutes. Typical data are displayed in figure 4. Data processing is performed subsequently as follows. First the analysis of the instantaneous photon counting rate (computed as the number of photons detected over a 40 μs long sliding acquisition window) allows us to detect the passage of successive droplets across the excitation laser, with a higher counting rate (i.e. above a fixed threshold) inside droplets, and lower (below threshold) in between two droplets. Second, all the time-correlated, single photon detection events corresponding to a given droplet are assembled in one histogram representing the fluorescence decay kinetics of this very droplet. At a typical MHz photon counting rate inside the droplets, fluorescence decay curves consisting of a few thousand photons in total may be collected per droplet, at a droplet circulation rate exceeding 300 per second. An automatic fitting procedure is programmed to extract the FLT of all single-droplet histograms, by performing a single parameter monoexponential fit (nonlinear least-square minimizing routine implemented with Matlab). More precisely, the fitting function is an exponential decay ( with N the total number of photons in the histogram, and τ the FLT), convolved with the instrument response function, determined separately and modeled analytically by a gaussian function of 80 ps full width at half maximum. An example of such a fit is displayed in figure 4(c).
with N the total number of photons in the histogram, and τ the FLT), convolved with the instrument response function, determined separately and modeled analytically by a gaussian function of 80 ps full width at half maximum. An example of such a fit is displayed in figure 4(c).
Figure 4. Implementation of TCSPC detection in microfluidic droplets containing 1 μM substrate and 1 nM enzyme and illustration of the data processing. (a) Instantaneous photon detection rate as a function of time (black line). The passage of the successive droplets in the focused laser spot is automatically detected (red line). In this example, the fluorescence acquisition time is ∼3 ms per droplet, which is the droplet circulation time across the laser pulse. (b) Fluorescence decay histograms of three individual droplets, composed of ∼2300 photons each. (c) Sum of the histograms of ten identical, successive droplets with a mono-exponential fit (red line). This corresponds to a cumulated 30 ms fluorescence acquisition time, meaning a  increase in the FLT precision in the case of a monoexponential decay (see text).
increase in the FLT precision in the case of a monoexponential decay (see text).
Download figure:
Standard image High-resolution imagePrevious investigation of the physical limitations of the photon counting technique in these very high throughput conditions allowed us to make several conclusions [36]. First, as a very general property of single photon counting, the measured FLT is accurate as long as the probability that more than one photon hitting simultaneously the SPAD remains low. If this condition is not fulfilled, the measured fluorescence decay histogram is distorted, and the FLT underestimated. This is known as the pile-up effect [52, 53]. In the present conditions, it can be shown to remain negligible [36] at photon detection rates lower than one tenth of the laser repetition rate typically, i.e. 2 MHz here. Second, due to this fundamental limitation in photon counting rate, the total number of photons detected during a typical 3 ms exposure time remains relatively small (few thousands), and consequently the photon detection noise (so-called 'shot noise') is the origin of the weak signal-to-noise ratio observed in the histograms of individual droplets (figure 4(b)). This is therefore also the dominant source of imprecision on the FLT, determined by the monoexponential fit of the noisy histograms. Third and most importantly, this shot noise limits the relative precision on the FLT value to  at best, with N the total number of photons in the histogram [54, 55]. This is a well-known consequence of the Poissonian statistics which characterizes the shot noise inherent to single photon detection. This implies in particular that for a monoexponential decay, measuring a FLT is in principle not less precise and does not require more photons than measuring a FLINT, since the latter is also characterized by the same
at best, with N the total number of photons in the histogram [54, 55]. This is a well-known consequence of the Poissonian statistics which characterizes the shot noise inherent to single photon detection. This implies in particular that for a monoexponential decay, measuring a FLT is in principle not less precise and does not require more photons than measuring a FLINT, since the latter is also characterized by the same  imprecision at best. With the present experimental set-up, we have shown that the actual relative precision on the measured FLT approaches very closely the ultimate value of
imprecision at best. With the present experimental set-up, we have shown that the actual relative precision on the measured FLT approaches very closely the ultimate value of  to within a factor of 1.2–1.5 [36]. Therefore accurate discrimination between two distinct FLT's corresponding to positive and negative control samples is achievable in a screening experiment, even at a very high throughput implying the detection of a relatively limited number of photons per sample. This is what we will demonstrate below in a proof of principle experiment based on a biologically relevant, enzyme activity assay.
to within a factor of 1.2–1.5 [36]. Therefore accurate discrimination between two distinct FLT's corresponding to positive and negative control samples is achievable in a screening experiment, even at a very high throughput implying the detection of a relatively limited number of photons per sample. This is what we will demonstrate below in a proof of principle experiment based on a biologically relevant, enzyme activity assay.
3.2. Application to the feasibility of an enzymatic activity assay
The enzyme activity assay was developed and validated previously [15, 18, 19]. It is based on the increase in the FLT of the PT14 probe which is initially quenched by collisions with the flexible tryrosine residue in the substrate. After trypsin has cleaved the substrate, the tyrosine is separated from PT14 and the probe's FLT is restored. Hence the increase of the FLT is the signature of the enzyme activity. As a test to demonstrate the feasibility of this assay by implementing TCSPC in the conditions illustrated in figures 3 and 4, we performed two successive experiments, where we measured the FLT of microdroplets circulating at a flow rate of about 300 per second. In the first one, the droplets were produced to contain the substrate solution at 1 μM concentration (see Material and Methods), by injecting the same solution in the three aqueous inlets. In the second experiment, a solution of 1 μM substrate and 1 nM enzyme was prepared about half an hour in advance such that the enzymatic reaction was initiated (see figure 1) before injection in the three aqueous inlets of the microfluidic chip. Hence, in these two experiments, each individual droplet is a new control sample to evaluate the FLT of the negative (first experiment) or positive (second experiment) control samples. Each acquisition run lasts for a few seconds only. By analyzing statistically the result of the FLT measurement in each individual droplet (sample), we quantify the precision with which the FLT of the negative and positive control samples may be determined in these very high throughput conditions, and very low sample volumes.
The fluorescence decay histograms obtained for individual droplets were composed of approximately 2300 photons and were fitted automatically as described above, with the FLT τ as a unique fitting parameter. Figure 5(a) displays the distribution of the FLT's measured on thousands of droplets in both experiments. More precisely, the FLT of the negative control sample (first experiment) is determined to be  ns with a RMS standard deviation of
ns with a RMS standard deviation of  ns (green distribution), while that of the positive control (second experiment) is
ns (green distribution), while that of the positive control (second experiment) is  ns with
ns with  ns (red distribution). The corresponding coefficients of variations (CVs) are thus no more than ∼3.6% in both cases. The feasibility of the enzymatic assay in these very high throughput conditions may be quantified by the so-called Z' factor [56]. In the present case of an assay based on a FLT measurement it writes:
ns (red distribution). The corresponding coefficients of variations (CVs) are thus no more than ∼3.6% in both cases. The feasibility of the enzymatic assay in these very high throughput conditions may be quantified by the so-called Z' factor [56]. In the present case of an assay based on a FLT measurement it writes:  with the notations introduced above. With as little as 3 ms exposure time per sample we already achieve Z' = 0.55, which exceeds 0.5 and therefore demonstrates the feasibility of the assay in these conditions.
with the notations introduced above. With as little as 3 ms exposure time per sample we already achieve Z' = 0.55, which exceeds 0.5 and therefore demonstrates the feasibility of the assay in these conditions.
Figure 5. (a) Distribution of the FLT measured on individual droplets circulating at a flow rate of 300 s−1 across the excitation laser beam. The droplets contain 1 μM substrate with (red) or without (green) addition of 1 nM enzyme. The CVs are 3.6% resulting in Z' = 0.55 (see text). (b) Summing individual droplets histograms 10 by 10 is equivalent to a 10-fold increase of the effective droplets exposure time. Repeating the same statistical analysis on this second set of histograms containing ten times more photons on average reduces the CVs to 1.2% and yields Z' = 0.85.
Download figure:
Standard image High-resolution imageFinally, we note that since the precision on the FLT determination is limited by Poisonian photon shot noise [36], a 10 fold increase in the sample exposure time should improve by  the CVs. This is easily confirmed by summing the histograms of ten successive droplets (all droplets are identical in a given experiment), before performing the automatic fit of the resulting histograms. The results are displayed in figure 5(b) and confirm that the CVs decrease to 1.2% as expected. By this analysis, we show that extending the exposure time to 30 ms, i.e. reducing the throughput to 1800 samples per minute which is still very high, the assay can be performed with a Z' factor as large as Z' = 0.85.
the CVs. This is easily confirmed by summing the histograms of ten successive droplets (all droplets are identical in a given experiment), before performing the automatic fit of the resulting histograms. The results are displayed in figure 5(b) and confirm that the CVs decrease to 1.2% as expected. By this analysis, we show that extending the exposure time to 30 ms, i.e. reducing the throughput to 1800 samples per minute which is still very high, the assay can be performed with a Z' factor as large as Z' = 0.85.
4. Discussion and conclusion
In assessing the performances of TCSPC for high-throughput FLT detection, it is important to remember that in a TCSPC experiment the precision achieved on the measured FLT theoretically scales as  where N is the total number of photons in the entire histogram (rather than the number of photons in the dominant bin). More precisely [54], it is in principle enough to detect 104 photons to achieve a relative precision (i.e. coefficient of variation, CV) of 1% on the FLT of a monoexponential decaying fluorescent probe. As in our previous work [36], the above results confirm that in a realistic, simple experimental implementation (home-made microscope), a CV value of 1.2% (see figure 5(b)) can easily be achieved by collecting about 2 × 104 photons per histogram (as in figure 4(c)) in a cumulated 30 ms exposure time. This corresponds to an 800 kHz photon counting rate, which is low enough, in the present experiment performed with a 20 MHz laser repetition rate, to prevent any significant pile-up effect that would affect the accuracy of the determined FLT [36].
where N is the total number of photons in the entire histogram (rather than the number of photons in the dominant bin). More precisely [54], it is in principle enough to detect 104 photons to achieve a relative precision (i.e. coefficient of variation, CV) of 1% on the FLT of a monoexponential decaying fluorescent probe. As in our previous work [36], the above results confirm that in a realistic, simple experimental implementation (home-made microscope), a CV value of 1.2% (see figure 5(b)) can easily be achieved by collecting about 2 × 104 photons per histogram (as in figure 4(c)) in a cumulated 30 ms exposure time. This corresponds to an 800 kHz photon counting rate, which is low enough, in the present experiment performed with a 20 MHz laser repetition rate, to prevent any significant pile-up effect that would affect the accuracy of the determined FLT [36].
When comparing the performances of the DWR and TCSPC techniques for high throughput FLT detection, we note that DWR does not perform better in terms of acquisition speed, since 50 ms are required to achieve CV = 1.64% when measuring the 4 ns FLT of Rhodamine 6G with the DWR technique [22]. Importantly, while the limitation on the FLT precision and its physical origin are well characterized in the case of TCSPC (due to Poissonian photon shot noise, therefore implying the  scaling law), it is not the case for DWR, where the analog nature of the acquisition scheme introduces additional, electronic sources of noise. Their influence on the FLT precision is not straightforwardly characterized.
scaling law), it is not the case for DWR, where the analog nature of the acquisition scheme introduces additional, electronic sources of noise. Their influence on the FLT precision is not straightforwardly characterized.
In terms of detection sensitivity, the single photon detection technique is certainly unparalleled. It enables detection of much lower sample volumes and molecule quantities, ultimately down to the single molecule level. In the present conditions, we emphasize that, the excitation laser average power is as low as 50 μW despite the use of a not very bright PT14 chromophore. This certainly contributes making the TCSPC approach very cost-effective. What is more, considering that the sample volumes are as low as 200 pL, the results above demonstrate that the enzymatic activity assay is feasible with substrate (respectively enzyme) quantities as low as ∼200 attomol (respectively 0.2 attomol) per sample, and at a throughput as high as 300 samples per second. This underlines the extreme sensitivity of TCSPC for FLT determination, even at very high throughput conditions.
Importantly, we stress that a major interest of using droplet microfluidics for the present demonstration is to realize very challenging conditions to assess the performances of TCSPC for very sensitive and accurate FLT sensing at very high throughtput. Certainly the FLT detection technology proposed here and the conclusions of this work would be directly transposable to microplate readers which realize much less challenging conditions in terms of throughput and required sensitivity. Conversely, the present demonstration calls for the implementation of droplet sorting based on FLT detection in DmF chips. This would require on-the-fly processing of single photon detection events in order to (i) detect automatically the passage of successive droplets and (ii) determine the FLT in real-time. To the best of our knowledge, no available commercial TDC allows such real-time data processing which requires hardware programming to achieve the desired processing rates [38, 57].
In conclusion, the technical implications of the present work are twofold. First it demonstrates that FLT detection is achievable with good precision in very small volume samples and chromophore quantities at high detection throughput. This paves the way to replacing FLINT by FLT detection for microfluidic applications, including e.g. fluorescence-assisted droplet or cell sorting [6, 58]. Second it offers a FLT detection which is orders of magnitude more sensitive than DWR and equally (if not more efficiently) applicable to high-throughput acquisition and HTS in particular, also in conventional microplate readers.
Future development of the TCSPC technique towards improved sensitivity, better FLT precision or shorter exposure times will rely on the ability to increase the maximum photon counting rate. The later is presently limited to the few-MHz range, due to limitations in the SPAD's electrical operation which imposes a typical 40–50 ns dead time. However, present CMOS technology enables integrating in a single microelectronic chip multiple, independent channels for time-correlated, single-photon detection in order to parallelize the acquisition of the fluorescence emission of a given sample, so as to enhance significantly the effective photon counting rate [59, 60]. We are exploring the implementation of this technology for the present application.
Acknowledgments
This work is supported by the Région Alsace via the Contrat doctoral No. 493-14-C22, the Institut Carnot MICA via the 'TR-Fluofluidics' project, and the French Agence Nationale de la Recherche via the ANR-15-CE11-0006 'PICO2' project. The above funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no conflicting interests exist.