Abstract
Magnesium alloys are regarded as potential biodegradable load-bearing biomaterials for orthopedic applications due to their physico-chemical and biomechanical properties. However, their clinical applicability is restricted by their high degradation rate, which limits the physiological reconstruction of the neighbouring tissues. In this work, a multifunctional coating architecture was developed on an AZ31 alloy by conjoining an anodization process with the deposition of a polymeric-based layer consisting of polyether imine reinforced with hydroxyapatite nanoparticles, aiming at improved control of the corrosion activity and biological performance of the Mg substrate.
Anodization and coating protocols were evaluated either independently or combined for corrosion resistance and biological behaviour, i.e. the irritation potential and angiogenic capability within a chicken chorioallantoic membrane assay, and bone tissue response following tibia implantation within a rabbit model.
Electrochemical impedance spectroscopy (EIS) analysis showed that coated Mg constructs, particularly anodized plus coated with AZ31, exhibited excellent stability compared to the anodized alloy and, particularly, to the bare AZ31. Microtomographic evaluation of the implanted samples correlated with these degradation results. Mg constructs displayed a non-irritating behaviour, and were associated with high levels of vascular ingrowth. Bone ingrowth neighbouring the implanted constructs was observed for all samples, with coated and anodized plus coated samples presenting the highest bone formation. Gene expression analysis suggested that the enhanced bone tissue formation was associated with the boost in osteogenic activity through Runx2 upregulation, following the activation of PGC-1α/ERRα signaling.
Overall, the developed multifunctional coatings appear to be a promising strategy to obtain safe and bioactive biodegradable Mg-based implants with potential applications within bone tissue.
Export citation and abstract BibTeX RIS
Introduction
Magnesium (Mg) alloys are regarded as potential materials for orthopedic-related applications, particularly as biodegradable load-bearing implants (Staiger et al 2006). Compared to the use of traditional non-resorbable titanium, stainless steel and cobalt-chromium-based materials, the application of Mg alloys obviates the need for a second surgical intervention for implant removal and minimizes stress shielding effects, due the similarity of the elastic properties of these materials to the ones of bone tissue (Staiger et al 2006, Witte et al 2008b). Mg alloys have also shown adequate cytocompatibility (Huan et al 2010, Hänzi et al 2010) and biocompatibility (Hänzi et al 2010, Kraus et al 2012) within cell culture studies and implantation in animal models, respectively. Within in vivo trials, Mg implants were found to support an enhanced apposition of mineralized tissue (Kraus et al 2012) and even outperformed titanium implants in terms of osseointegration and bone–implant interface strength (Castellani et al 2011).
However, the clinical applicability of Mg alloys is limited by their rapid corrosion rate in an electrolytic aqueous environment, leading to detrimental interactions with biological organisms. Further, while galvanic contact corrosion established with other conducting materials can be lessened, most alloy impurities or secondary phases can lead to internal galvanic corrosion with destructive effects, thus affecting the mechanical properties of the implant before the completion of tissue healing (Xu et al 2008a). Of additional relevance, hydrogen is formed during Mg corrosion and when the physiological threshold of hydrogen saturation is exceeded, gas accumulates in formed cavities, which limits the physiological reconstruction of the neighbouring tissues. An alkaline pH shift in the vicinity of the corroding surface may also pose a limitation for an adequate biological response (Song 2007).
Within this context, the corrosion resistance of Mg alloys must be controlled and tailored for clinical application, and this can be achieved by different approaches including alloying (Wu et al 2012) and modification of the surface structure (Tomozawa and Hiromoto 2011, Yang et al 2011). Elements such as calcium, lithium, zinc, zirconium, rare earth metals, or aluminum are most commonly used for alloying (Witte et al 2008b). However, alloying can be a demanding process due to the low solubility of distinct elements in Mg, and despite the improvement attained in corrosion resistance, some elements may cause adverse biological effects (Zhao and Zhu 2015).
Coatings, including conversion, organic, inorganic and hybrid ones, have been reported to be of high significance, and an attractive approach to improve corrosion resistance (Hornberger et al 2012). Coatings can be obtained by numerous techniques and are aimed at establishing a physical barrier between the substrate and the corrosive micro-environment. In this regard, a previous approach was developed embracing a deposited hybrid coating consisting of polyether imide (PEI) and hydroxyapatite (HA) nanoparticles (Zomorodian et al 2013). The developed hybrid coating was found to lessen the corrosion rate of AZ31 magnesium alloys and to improve the adhesion and proliferation of seeded osteoblastic cells (Zomorodian et al 2013).
More recently, a combined approach of composite coatings, and conjoining chemical and physical treatments, have been assayed and found to further control the degradation of Mg substrates, as assayed by EIS (Wang et al 2012). However, data on the biological behaviour of composite coatings is broadly sparse as, to the best of our knowledge, no previous characterization of in vitro or in vivo models has been developed.
In the present study, a multifunctional coating architecture was developed by conjoining an anodization layer with a polymeric-based top layer (PEI reinforced with HA nanoparticles), aiming at improved control of the corrosion activity and biological performance of a Mg alloy, AZ31 alloy, used as a substrate. Anodization and coating protocols were evaluated either independently or combined regarding their biological performance, i.e. the irritation potential and angiogenic capability within a chicken chorioallantoic membrane (CAM) assay, and bone tissue response following tibia implantation within a rabbit model.
Experimental section
Mg constructs preparation
Within the present study, four experimental groups were prepared and characterized: bare AZ31, anodized AZ31, coated AZ31 and anodized plus coated AZ31.
Bare AZ31 was obtained from a commercially available AZ31 Mg-based alloy (Goodfellow, Inc.), which was polished with 2100 grit SiC paper. The AZ31 samples were degreased in ethanol. A set of samples was pre-treated for 1 h in 12% HF, creating a conversion layer for the coating deposition.
The samples to be anodized were first immersed in 1.0 M NH4F solution, at 80 °C, for 30 min to deposit a MgF2 thin layer (less than 100 nm). The anodization protocol was then developed in a two-stage process: KOH 100 g l−1 + Na3PO4 100 g l−1 (DC current 5.2 A dm−2, voltage up to 30 V, 10 min, 20–25 °C), followed by KOH 6 g l−1 + NaF 13 g l−1 (DC current 3.7–1.5 A dm−2, voltage up to 180 V, 10 min, 20–25 °C), according to a previous report (Kwiatkowski et al 2015). The thickness of the developed anodic film was found to range within an 23–25 µm interval. The absence of oxide coating located under the electric contact terminal to the power supply, or at the edge of the sample, was repaired by selective anodizing, carried out using KOH 100 g l−1 + Na3PO4 100 g l−1 1 min, voltage up to 150 V, followed by KOH 6 g l−1 + NaF 13 g l−1 1 min, voltage up to 150 V.
The polymeric-based coating was synthesized by mixing PEI in N,N-dimethylacetamide, as a solvent, in concentrations of 15 wt.%, and HA nanoparticles in concentrations of 2 wt.%. The mixture was stirred for 24 h, at 50 °C, to obtain a stable and homogeneous solution. Diethylenetriamine (DETA) was added to the formulation at 0.3 wt.%, and was further mixed for one hour. The coating was applied by dip coating and the coated samples were cured at 150 °C for 2 h to promote polymerization and removal of solvent excess. Details on the coating synthesis and applications have been previously published (Zomorodian et al 2013). The coating was applied on bare AZ31 samples, referred to as coated AZ31, or on anodized samples, referred to as anodized plus coated AZ31.
Mg constructs physico-chemical characterization
Microstructure characterization
The chemical composition of AZ31 was determined using wavelength dispersive x-ray fluorescence spectroscopy, using BRUKER S4 Explorer equipment. Samples were polished with standard metallographic techniques, in which a mixture of ethanol and glycerin were used as lubricant. Samples were washed in an ultrasonic cleaner in a two-step process with ethanol and isopropanol.
Characterization of the bare AZ31, anodized AZ31, coated AZ31, and anodized plus coated AZ31 samples was conducted using scanning analytical electron microscopy (Hitachi SU 70) with energy-dispersive x-ray spectroscopy capability. Analyses were conducted on both the surface and at the materials' cross-section (n = 6).
Characterization of corrosion resistance
The corrosion behaviour of the materials was evaluated by electrochemical methods carried out in a simulated body fluid (SBF) solution, with a previously reported composition (Bohner and Lemaitre 2009). The corrosion resistance was assessed by EIS. EIS measurements were conducted using a three-electrode cell with the sample as the working electrode, a platinum mesh as a counter electrode and saturated calomel electrode as reference electrode. Experiments were performed at the open corrosion potential by applying a sinusoidal signal of 10 mV in amplitude, over a frequency range from 105 Hz to 10−3 Hz, spaced logarithmically using a Solartron1287/1260 system (n = 6). The corrosion resistance was studied up to 90 d of immersion, except for the bare AZ3, which was monitored for a much shorter time due to heavy corrosion activity.
Mg constructs biological evaluation
In vivo characterization within a CAM assay
The CAM is a highly vascularized extraembryonic membrane, which performs multiple functions during embryonic development of some amniotes (Nowak-Sliwinska et al 2014). The CAM, due to its visibility, accessibility and rapid developmental growth has been used as a robust experimental platform to address tissue response and blood vessel studies in a wide range of validated preclinical in vivo assays (Nowak-Sliwinska et al 2014). Within this study, two chicken CAM-based assays were developed in order to evaluate the irritation potential and the angiogenic response of the developed Mg constructs, according to the described protocols.
Fertile White Leghorn chicken eggs, obtained from commercial sources, were maintained in a rotating incubator in standard conditions (37 °C in 60% relative air humidity, rotated hourly). On day 3 of incubation, 3 ml of albumen was withdrawn using a 21-gauge needle through the large blunt end of the egg and a window was made in the eggshell to allow access to the CAM. The window was covered with a piece of tape to prevent dehydration and the eggs were placed in the incubator and maintained for a further 6 d.
Assessment of the irritation potential—CAM assay
48 fertile White Leghorn chicken eggs, incubated and prepared as previously described, were used for the evaluation of the irritation potential. After 9 d of incubation, cylindrical Mg constructs (1.6 mm in diameter and 7 mm in length) of the four distinct groups were grafted over the CAM (n = 8, 1 sample per egg). The irritation potential was scored in accordance to the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) guidelines. Briefly, following implant grafting, the CAM was observed for a period of 300 s, and monitored for the development of vascular irritation alterations (i.e. vessel lysis, hemorrhage and coagulation). These described test endpoints were scored in a numerical time-dependent manner according to the references shown in table 1. Results were then summed to give a single numerical value indicating the irritation potential of the tested construct on a scale ranging from nonirritating (0) to severely irritating (from 9, upwards). As an internal control, negative (0.9% NaCl) and positive (0.1 N NaOH) controls were used, in accordance with ICCVAM guidelines. At the 300 s endpoint, embryos and their membranes were fixed in ovo with 4% paraformaldehyde at −80 °C, for 10 min. The implanted Mg constructs and the underlying and immediately adjacent CAMs were then cut and ex ovo images were captured.
Table 1. Scoring scheme for irritation testing with the hen's egg test (HET)-CAM test method.
| Effect | Score | ||
|---|---|---|---|
| 30 s | 120 s | 300 s | |
| Lysis | 5 | 3 | 1 |
| Haemorrhage | 7 | 5 | 3 |
| Coagulation | 9 | 7 | 5 |
Assessment of the angiogenic response—CAM assay
40 fertile White Leghorn chicken eggs, incubated and prepared as previously described, were used for the evaluation of the angiogenic response. Following 9 d of incubation, circular Mg constructs (4 mm diameter and 2 mm thickness) of the four distinct groups were grafted over the CAM (n = 8, 1 sample per egg), and were evaluated for vessel quantification in the vicinity of the implant. A negative control (filter paper impregnated with 0.9% saline) was used. Prepared Mg constructs and controls were grafted and left for 3 d in order to address the effect of the constructs in the new vessel formation process. At this time point, in ovo images were captured for each implanted CAM and the macroscopic evaluation of the vasoproliferative response was carried out by quantifying the convergence of blood vessels towards the graft.
In vivo orthotopic implantation in rabbit tibia
All animal experiments were conducted under a local Institutional Animal Care and Use Committee (IACUC) approved protocol, in accordance with the national and European legislation for experimental animal research.
Surgical procedure
12 adult male New Zealand white rabbits (mean weight: 3.8 ± 0.42 kg) were acquired from a certified vendor. For the rabbit implantation assay, cylindrical Mg constructs (1.6 mm in diameter and 7 mm in length) of the four distinct groups were used. All rabbits received four Mg implants in total, two on the left tibia and two on the right tibia, which were randomly distributed. Six rabbits were endorsed for the postoperative follow-up in one of the following groups: 2 and 6 weeks (n = 12).
Prior to the surgical implantation, animals were pre-medicated with intramuscular injections of 25 mg kg−1 ketamine and 1 mg kg−1 midazolam. Buprenorphine (0.03 mg kg−1) was used for analgesia. General anesthesia was induced by 1 mg kg−1 propofol IV and, after endotracheal intubation, anesthesia was continued with isoflurane (2.5–3.5%/oxygen mixture, 1.5–2.0 l min−1). During the entire surgical procedure sterile saline was administered at 10 ml kg−1 h−1. Following the confirmation of the anesthetic plane, trichotomy was conducted on both legs that were aseptically prepared for surgery. An anteromedial approach to the proximal tibia was conducted. Two 1.6 mm diameter holes were drilled into the medial proximal tibia and the previously randomized Mg constructs were press-fitted into place. The soft tissues were then closed in layers using absorbable sutures. Post-operative x-rays ensured the correct position of the implant and were used as a follow-up control for forming gas cavities. The hind limbs of each animal were clinically examined until euthanasia in order to detect the formation of subcutaneous gas cavities originating from the corroding implants. During the postoperative period, rabbits were allowed to move freely in their cages and an analgesic regimen with buprenorphine was maintained during the first week.
In order to address systemic toxicology effects, histopathological evaluation of the liver and kidney was conducted. Furthermore, the biological response of the implants within the grafted tibias was evaluated by microtomographic analysis, addressing the characterization of the implant degradation process and the disclosure of the bone formation process in the vicinity of the implanted constructs.
Microtomographic determination of the residual implant volume and bone tissue regeneration
Microtomographic analyses were conducted in a commercially available cabinet cone-beam microCT, (µCT 100, SCANCO Medical AG, Brüttisellen, Switzerland) with the following parameters: voxel size of 17.2 µm, x-ray voltage of 90 kVp, intensity of 88 µA and an integration time of 400 ms.
The residual implant volume was analysed using a previously described protocol (Kraus et al 2012). Briefly, greyscale values were initially segmented and a 3D volume growing method was conducted in order to allow the separation of the magnesium construct from the surrounding bone matrix. Threshold levels of 2180 Hounsfield units (HU) and 2578 HU were used for the identification of bone and magnesium, respectively.
Microstructural measures of the neighbouring bone tissue, including bone volume (BV), total volume (TV) and the ratio of bone volume to total volume (BV/TV) was quantified. The computation of these structural measures has previously been described (Durão et al 2014).
Gene expression analysis of the regenerated bone tissue
The bone tissue within the vicinity of the implanted Mg pins was harvested with a trephine (n = 3). The samples were then frozen in liquid nitrogen and mechanically ground to powder. The total RNA was isolated using the TRIzol reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's protocol. The concentration of the total RNA was determined using a spectrophotometer. cDNA was synthesized from 0.5 µg of total RNA using a commercial first-strand cDNA synthesis kit (QIAGEN, Hilden, Germany). Reverse transcription PCR was performed in triplicate for each sample, using primers specifically for amplification of rabbit Runt-related transcription factor 2 (Runx2), osteopontin (OPN), osteocalcin (OC), peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), estrogen-related receptor alpha (ERRα), and peroxisome proliferator-activated receptor-gamma coactivator 1 beta (PGC-1β) genes. Sequences of the primers used are presented in table 2. Real-time PCR reactions were performed using SYBRGreen in a 7900HT fast real-time PCR System (Applied Biosystems, Darmstadt, Germany). To categorize specific PCR products, a melting curve was obtained at the end of each run. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels were used to normalize procedures and relative mRNA expression was presented in comparison to the baseline levels attained in bare AZ31 samples.
Table 2. Sequences of the primers used for real-time PCR analysis.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | TCACCATCTTCCAGGAGCGA | CACAATGCCGAAGTGGTCGT |
| RUNX2 | CCTTCCACTCTCAGTAAGAAGA | TAAGTAAAGGTGGCTGGATAGT |
| OPN | GCTCAGCACCTGAATGTACC | CTTCGGCTCGATGGCTAGC |
| OC | CAGGGGTCAGGTGGTTGTAG | CCGAGGGAGAGGAGGGAATA |
| PGC-1α | CCACAGCACGGGAAAACTTG | GGTAGTTGGCTCTCGGCAAT |
| ERRα | GTTCCCTGTGGGTGTCATGG | TGCCTAGCGGTCTTTCCAAG |
| PGC-1β | TGACCTCACACGGCTTTCTC | ACATTCCTGCTGGGGATTGG |
Statistical analysis
All data were collected and stored in a database. Subsequently, analyses were performed using the statistical analysis program (SPSS® v.16.0, Statistical Package for the Social Sciences) and a significance level of 5% was considered (p < 0.05). Hypotheses on the distribution of continuous variables between two independent samples were tested using one-way analysis of variance or a non-parametric test (Mann–Whitney), when appropriate. The normality distribution of variables was tested using the Shapiro–Wilk test.
Results and discussion
Mg construct morphology and corrosion behaviour
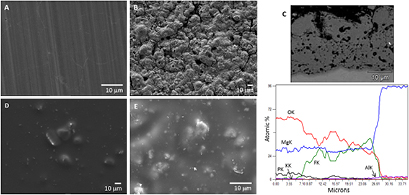
Data regarding the morphological characterization of the developed constructs is presented in figure 1. The top view of bare AZ31 shows a polished surface (figure 1(A)) compared to the rough appearance typical of the anodized alloy (figure 1(B)). The anodized coating exhibits a structure consisting of an outer porous layer, mainly composed of magnesium, oxygen and phosphorus. At the metal coating interface, an inner layer composed of magnesium, oxygen and fluorine was identified (figure 1(C)). Taking into account the elemental profile of this coating, the presence of magnesium oxides, hydroxides and phosphates in the outer layer, and magnesium fluoride together with magnesium oxides in the inner part of the coating, may be expected (Kwiatkowski et al 2015). The coated samples exhibited a crack free and smooth surface, with the presence of agglomerated hydroxyapatite particles (figure 1(D)). Previous studies have shown that the sizes of these agglomerates were found to be less than 1 µm, being four times below the maximum coating thickness and fully covered by the polymeric film (Zomorodian et al 2013). By contrast, anodized plus coated AZ31 samples showed a rougher morphology, although with similar pattern, i.e. hydroxyapatite agglomerates fully integrated into the smooth polymeric coating (figure 1(E)). As previously demonstrated the presence of these agglomerates helps to tailor the corrosion protectiveness of the coating.
Figure 1. Representative SEM images of bare AZ31 (A), anodized AZ31 (B), cross section of anodized AZ31 revealing the anodic oxide coating layer and the elemental content (C), coated AZ31 (D) and anodized plus coated AZ31 (E).
Download figure:
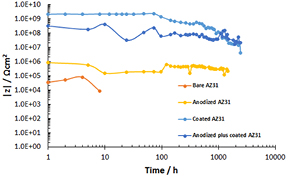
Standard image High-resolution imageThe corrosion process was monitored over time by EIS and the evolution of the total impedance of the system, determined at low frequency, was used as an indicator of the corrosion resistance as reported in a previous work (Zomorodian et al 2013). The evolution of the impedance modulus |Z| at low frequencies (0.01 Hz), during the immersion period is depicted in figure 2. The results show that at early stages the highest impedance values were recorded for the coated AZ31 samples. During exposition to SBF there was a slight decrease in |Z| values with time, suggesting a weakening of the barrier properties. Anodized plus coated AZ31 exhibited slightly lower impedances at the beginning of exposition, and also minor changes in |Z| values were noticed during immersion in the SBF solution, thus indicating high stability of the protective system throughout the assayed time. The coated samples revealed |Z| values much higher than those measured for the anodized sample and several orders of magnitude higher than the bare AZ31. The reference bare AZ31 showed quite low impedance and the test was terminated after 48 h due to the strong corrosion activity. At the end of the test period, impedance values were found to be much higher for the coated samples compared to the anodized ones. The results evidence the fact that the presence of the PEI coating significantly enhances the protective behaviour of the anodic oxide.
Figure 2. The evolution of the impedance modulus |Z| at low frequencies (0.01 Hz) during the immersion time of developed AZ31 constructs in SBF solution.
Download figure:
Standard image High-resolution imageIrritation reactivity test—CAM assay
Irritation testing aims to disclose the capacity of the tested substance/material to establish a reversible local inflammatory reaction caused by the activation of innate immunological mechanisms. Currently, test methodologies for irritation assessment include the traditional in vivo animal evaluation within the skin and/or mucosal sites, as well as alternative models, which have been developed in order to minimize animal use and distress. The CAM assay has been routinely used for irritation assessment, with adequate validity (Silva et al 1992). This assay is an extension of the traditional chicken embryo model used within embryo-toxicological and biomaterial evaluation, and discloses the assessment of vascular alterations verified within the CAM following construct implantation (Silva et al 1992, Vinardell and Mitjans 2008). The assay addresses the appearance of irritative reactions (i.e. hyperemia, hemorrhage and coagulation/clotting), as a response to the exposition of the biological membrane to the test sample at predetermined time points (Barile 2010). Several works have concluded that adverse effects on the CAM can be highly correlated with the attained in vivo alterations, thus supporting the validity of the assay to predict severe and/or irreversible alterations on biological tissues (Barile 2010).
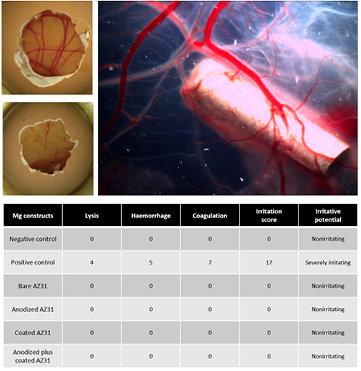
Data on the evaluation of the irritation potential using the CAM assay is presented in figure 3. Representative micrographs of the grafted CAM, with the negative and positive controls, and the implanted Mg constructs are depicted. The negative control (figure 3(A)) shows the development of a dense vascular network, without evidence of adverse alterations, whereas the positive control (figure 3(B)) shows vessel lysis, hemorrhage and coagulation. By contrast, implanted magnesium constructs were found not to alter the vascular network structure, in a similar way to the negative control (figure 3(C)). Quantitative data, based on the scoring algorithm, is presented in the table (figure 3, bottom), thus corroborating the non-irritating potential of assayed Mg coatings.
Figure 3. Representative in ovo CAM images of (A) negative control and (B) positive control. (C) Representative ex ovo micrograph of the CAM grafted anodized plus coated AZ31 sample. Note the absence of vascular alterations to the vascular network embracing the implant. The scale bar corresponds to 1.5 mm. Table: quantitative data on the irritation potential, based on the scoring algorithm.
Download figure:
Standard image High-resolution imageThe irritation potential of different magnesium alloys has been previously evaluated within experimental animal models, substantiating the non-irritating behaviour of these materials (Witte et al 2008a). Also, the long term implantation of Mg alloys was found not to induce significant changes in efferent lymph node dynamics, and to even induce a lower immunological reaction than that verified for the implantation of routine resorbable (i.e. polylactic acid) and non-resorbable (i.e. titanium) biomaterials (Bondarenko et al 2011). Some works have also addressed the irritation potential of the polymer used for the additive coating, PEI, converging to the idea that it possesses non-irritating properties. PEI was found to have neither stimulatory nor inhibitory effects on early immune mechanisms, such as complement activation, generation of reactive oxygen species, pro-inflammatory cytokine production (Roch et al 2012, 2014), and not to alter the survival and functionality of dendritic cells—the pivotal players in antigen presentation to T cells within the adaptive immune response (Roch et al 2013). Overall, data supports the non-irritating potential and low immunogenic capability of the tested alloy and the developed coatings, in accordance with the attained qualitative and quantitative CAM test results.
Angiogenic response—CAM assay
Angiogenesis, the formation of new blood vessels from pre-existing structures, is a central event within the host response to an implanted biomaterial, being a determinant for integrative tissue healing, with particular relevance within bone tissue regeneration (Hankenson et al 2011). The CAM model is widely used in angiogenesis-related research, being routinely conducted for the assessment of the angiogenic potential of developed biomaterials (Zwadlo-Klarwasser et al 2001, Azzarello et al 2007). In the present assay, magnesium constructs were implanted over the CAM on the tenth day of embryonic development, as between the ninth and twelth days of embryogenesis, the CAM reaches its maximum vascularization potential (Borges et al 2003).
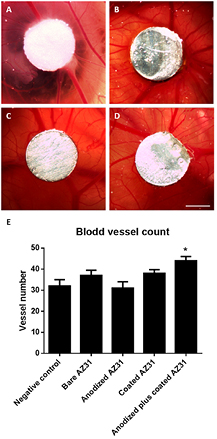
Results regarding the assessment of the angiogenic response to the developed magnesium constructs are presented in figure 4. Representative CAM micrographs of the negative control and grafted Mg constructs are shown in figures 4(A)–(D), while data on the quantification of the blood vessels converging toward the implanted materials is shown in figure 4(E). Microscopic evaluation within the assayed period revealed that magnesium constructs kept their structural integrity, with no evidence of coating or substrate fragmentation or degradation, as shown by in ovo micrographs. Further, Mg constructs were effectively integrated within the chorioallantoic membrane, as an adequate vascular sprouting with vessel ingrowth into the implanted substrates was verified, and no significant alterations to embryonic development were acknowledged. The quantification of blood vessel ingrowth towards the implants showed that both bare AZ31 and the anodized compositions performed in a similar way to the control. Coated and anodized plus coated conditions presented an induced response, which was significantly higher in the latter.
Figure 4. Representative in ovo CAM images of bare AZ31 (A), anodized AZ31 (B), coated AZ31 (C) and anodized plus coated AZ31 (D) grafted for 3 d on CAM. The scale bar corresponds to 0.75 mm. (E) Number of blood vessels converging toward the implanted Mg constructs.
Download figure:
Standard image High-resolution imageData on the in vivo angiogenic capability of magnesium alloys is scarce as, to the best of our knowledge, only one study has focused on the biological characterization of the CAM following Mg alloy implantation. This study showed that AZ31 induced minimal interference with the CAM development, following up to 3 d of implantation (Douglas-Byrd 2013).The attained data seems to correlate well with cell culture studies, as the evaluation of medical grade Mg alloy extracts with human endothelial cells showed minimal cytotoxicity and a rapid restoration of normal cell behaviour, meeting the biosafety criteria for clinical application, according to the ISO guidelines (Mao et al 2013). In addition, when distinct strategies were used to modify the surface of magnesium alloys and improve corrosion resistance, either by anodization, coating and/or oxidation, an improved cellular response was attained, with enhanced cellular attachment, viability/proliferation and functional activity, thus supporting a prospective improved angiogenic response (Wei et al 2014, Zhao et al 2014, Mao et al 2015). In fact, the developed coating produced an adequate biological response and, in combination with the anodization procedure, significantly enhanced the vascular ingrowth. In this regard, the use of PEI has been welcomed for biomedical applications embracing vascular supply, as in vitro studies showed that this polymer supported the adhesion, proliferation and phenotypic establishment of seeded human endothelial cells (Tzoneva et al 2008), at the same time that its in vivo implantation reported the inductive formation of functional blood vessels within the implant vicinity (Haase et al 2015).
In vivo orthotopic implantation in the bone tissue
Implant degradation
Animals were implanted with load-bearing Mg constructs and, throughout the 6 week follow up period, no post-operative complications, signs of infection, or other adverse host reactions were identified. At adequate time points, animals were euthanized and a systematic necropsy was conducted, revealing that no evidence of pathological alterations was identified on internal organs. The biological response to the implanted magnesium constructs was then conducted on retrieved tibias.
Clinical examination of the implantation areas revealed the formation of subcutaneous gas cavities at 2 weeks of implantation, within the vicinity of bare AZ31 samples. These were further confirmed by x-ray imaging, in which radiolucent areas around the ends of the implanted pins were identified, as shown in online supplemental figure S1 (stacks.iop.org/BMM/11/045007/mmedia). Identified gas cavities were found to disappear with time and, at the 6 week time point, were no longer identified, either clinically or radiographically. No adverse effects due to the gas formation were observed. Further, no clinical or radiographic alterations were identified regarding the implantation of anodized or coated samples.
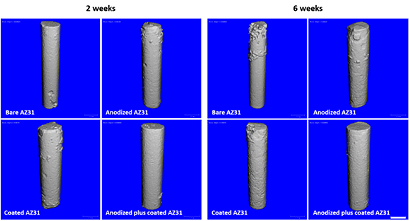
Microtomographic evaluation provides a representative pattern of the samples' degradation, as shown in figure 5. After 2 weeks of implantation, AZ31 implants exhibited surface alterations, with pit formation particularly concentrated at the implant ends. These alterations were also identified on anodized and coated AZ31 implants, though in a less significant way. By contrast, no significant surface alterations were identified on the anodized plus coating samples. At the 6 week time point, the structural integrity of the implanted constructs was maintained, regardless of the continued degradation process observed, particularly for the bare AZ31 samples. Anodized and coated samples showed evidence of pit formation, whereas the anodized plus coating sample did not display significant surface alterations. Quantitative data on the variation of the implant volume was in accordance with the analysed 3D reconstructions (table 3) —a significant volume loss, from 2 to 6 weeks of implantation was observed for AZ31 samples and, at the 6 week time points, uncoated and anodized implants presented a significantly lower volume than that of coated and anodized plus coating implants. Observed results on the microtomographic analysis correlate with conducted in vitro EIS analysis, thus supporting the improved corrosion performance of the anodized plus coating construct.
Figure 5. Microtomographic imaging of implanted AZ31 constructs at 2 and 6 weeks following implantation. The scale bar corresponds to 1 mm.
Download figure:
Standard image High-resolution imageTable 3. Implant volumes in percentage, as compared to a baseline volume.
| Samples | Implant volume (% relative to baseline) | |
|---|---|---|
| 2 weeks | 6 weeks | |
| Bare AZ31 | 93.78 ± 2.62 | 87.96 ± 1.95 |
| Anodized AZ31 | 92.76 ± 2.29 | 89.70 ± 2.54 |
| Coated AZ31 | 96.6 ± 2.76 |
94.67 ± 2.62 |
| Anodized plus coated AZ31 | 98.65 ± 2.62 |
97.14 ± 2.84 |
aSignificantly different from Bbare AZ31 at 2 weeks. bSignificantly different from Aanodized AZ31 at 2 weeks. cSignificantly different from Bbare AZ31 at 6 weeks. dSignificantly different from Aanodized AZ31 at 6 weeks. (p ⩽ 0.05).
The volume loss observed for implanted uncoated AZ31 samples is expected to accrue from the active corrosion process developing within the in vivo aqueous environment (Krause et al 2010). Briefly, magnesium is expected to react with water molecules leading to the formation of a magnesium hydroxide film that, in turn, reacts with chloride ions originating from the highly soluble form of magnesium chloride and hydrogen gas (Shaw 2003, Song 2005). Pitting of magnesium is verified in environments in which the chloride ion concentration exceeds 30 mmol l−1 and within physiological environments this anion is present at levels within the order of 150 mmol l−1 (Kirkland et al 2010). Chloride-mediated pitting corrosion may be further mediated by the presence of other ions such as phosphates and carbonates (Xin et al 2008). Previous reports on the in vivo degradation of AZ31 alloy revealed in situ gas accumulation throughout the early implantation period (Witte et al 2005, Montoya et al 2014, Iglesias et al 2015), as presently observed at the 2 week time point. However, gas cavities were found to disappear with longer implantation periods, a process expected to related to the gas diffusion into soft tissues, and found to occur without the impairment of the local tissue regeneration (Witte et al 2005, Iglesias et al 2015). In addition, at longer implantation times, the corrosion rate is expected to reduce due to the precipitation of magnesium oxides and hydroxides on the implant surface, which can, in turn, allow calcium phosphate precipitation, thus lessening the hydrogen release into the surrounding tissues and favouring the local cellular response (Witte et al 2005).
The anodized implants presented decreased corrosion activity, as no significant differences were found regarding implant volume between week 2 and 6 of implantation. Further, no gas cavities were identified, despite evidence of minor pit formation. Accordingly, anodization protocols were previously found to improve the corrosion and wear resistance of magnesium alloys, as anodic oxidation is a well established methodology for producing protective oxide films on the alloy surface (Song 2007, Salman et al 2010, Yang et al 2011). Nonetheless, within the biological environment, particularly in loading applications, anodization seems to offer a scarce protection to the substrate (Wang et al 2012). During anodization, gas evaporation may lead to micropore formation and the thin oxide/hydroxide film may be inefficiently uniform and inadequately cohesive to provide a long-lasting and effective protection (Wang et al 2012). Therefore, anodization protocols are broadly designed to be sealed or covered by other protective layers.
Within the present work, a coating protocol was developed to control the alloy degradation process. The coating was assayed on both bare and anodized AZ31 constructs and was found to reduce the corrosion of the substrate upon implantation. In the coated AZ31 construct, minor pit formation was identified, while within the anodized plus coated construct, no significant surface alterations were identified at either 2 or 6 weeks of implantation. Further, within the latter, no significant changes in the implant volume were found throughout the assayed periods, corroborating the highest corrosion resistance when both anodization and coating strategies were combined.
A wide range of polymeric coatings have been assayed for the enhancement of the biological response of magnesium alloys, thus aiming an improved corrosion resistance, abrasion and wear properties. Polymeric materials such as poly(lactic) acid, poly(lactic-co-glycolic acid), polycaprolactone and chitosan were found to provide effective corrosion protection and prospective enhancement of the biocompatibility of the used magnesium substrates (Xu et al 2008b, Wong et al 2010, Xu and Yamamoto 2012, Ostrowski et al 2013). In this work, a polymeric coating based on PEI, a hydrophobic, thermal and mechanical stable polymer with adequate film-forming properties, was used. Chemically, the presence of polar aromatic imide rings within the PEI structure was expected to improve the bonding to the substrate, as the reaction of the imide groups with bases opens the imide ring system and results in an improved metal/PEI adhesion, prospectively, with a self-healing capability (Conceicao et al 2010a). PEI has been previously found to enhance the corrosion resistance and lessen the degradation behaviour of magnesium substrates, as shown by EIS studies (Scharnagl et al 2009, Conceicao et al 2010b). The developed coating also included the addition of a low amount of DETA (0.3%) and hydroxyapatite nanoparticles (2%). Both were previously found to improve the in vitro coating performance in terms of corrosion resistance and barrier capability (Zomorodian et al 2013). Furthermore, the presence of adequately dispersed HA nanoparticles is expected to play a buffering role following crystal dissolution, which may reduce potential gradients between anodes and cathodes, and thus effectively reduce the corrosion rate (Snihirova et al 2010).
Overall, while both the anodization and coating protocols were found to improve the corrosion resistance in comparison to the bare alloy, the highest resistance and control of the implant degradation was observed when both processes were combined, offering an effective protection of the substrate, with negligible surface alterations and volume loss throughout the implantation period.
Bone tissue ingrowth
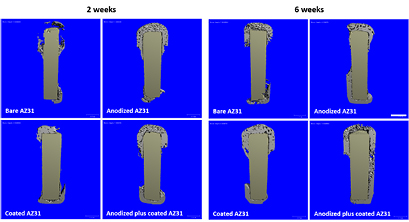
Data regarding the assessment of the bone ingrowth neighbouring the implanted Mg constructs are presented in figure 6 and table 4, based on results from the μCT investigation. A volume of interest 0.5 mm towards the implant surface was defined, and the bone ingrowth was quantified within this region. At 2 weeks, the mineralized tissue ingrowth was limited to the proximal and distal ends of the implanted constructs. By contrast, higher tissue formation was found within the volume neighbouring the anodized, coated and anodized plus coated implants, in comparison to the bare AZ31 alloy. Further, coated and anodized plus coated constructs presented higher bone tissue ingrowth, as comparing to the anodized preparation. At 2 weeks, an increased ingrowth of mineralized tissue was observed for all the assayed implant compositions, compared with the 2 week time point. Significant differences between implanted constructs were observed at the later time point, with coated AZ31 and anodized plus coated samples presenting the highest bone tissue formation; the latter was found to be the highest.
Figure 6. Microtomographic imaging of implanted AZ31 constructs at 2 and 6 weeks following implantation, addressing a cross-section of the implant with the newly formed bone tissue ingrowing a defined volume of 0.5 mm towards the implant surface. The scale bar corresponds to 1 mm.
Download figure:
Standard image High-resolution imageTable 4. BV/TV in a volume of interest 0.5 mm offset from the implant.
| Samples | BV/TV (%) | |
|---|---|---|
| 2 weeks | 6 weeks | |
| Bare AZ31 | 14.7 ± 3.6 | 8.3 ± 2.1 |
| Anodized AZ31 | 22.8 ± 4.1 |
28.6 ± 3.2 |
| Coated AZ31 | 28.6 ± 5.2 |
33.4 ± 5.3 |
| Anodized plus coated AZ31 | 29.8 ± 4.4 |
44.1 ± 3.4 |
aSignificantly different from bare AZ31 at 2 weeks. bSignificantly different from anodized AZ31 at 2 weeks. cSignificantly different from bare AZ31 at 6 weeks. dSignificantly different from anodized AZ31 at 6 weeks. eSignificantly different from coated AZ31 at 6 weeks (p ⩽ 0.05).
The bone response to implanted AZ31 alloy in experimental animal models has been previously evaluated by other authors, as discussed in the following. Regardless of the observed active corrosion, bone formation was observed at early implantation time points, revealing the apposition of mineral tissue bordering the implant, and supporting an active osteoblastic function in the vicinity of the implanted alloy (Witte et al 2005). Longer implantation periods also showed a local accumulation of bone forming cells within the vicinity of corroding AZ31 implants, with active osteoblastic recruitment and osteoid production (Willbold et al 2011). In the present study, although active bone formation was identified in the vicinity of corroding AZ31 implants, increased bone formation was observed when protective strategies were applied. In fact, the highest bone formation was attained when a combination of anodization and coating strategies were used, thus supporting the proposal that the effective modulation of Mg alloy corrosion further enhances bone tissue ingrowth.
Reports in the literature seem to converge to this idea, and several surface modification strategies have been used to modulate the alloy corrosion and enhance the biological performance. For instance, oxidation treatments, such as micro-arc oxidation, were found to control early implant degradation, inducing an adequate bone tissue response immediately after implantation (Fischerauer et al 2013, Lin et al 2013). Alternatively, ceramic (Xu et al 2009, Chai et al 2012, Shadanbaz et al 2014) and polymeric (Wong et al 2010, Kunjukunju et al 2013, Wang et al 2013) coatings have also been applied with success, minimizing the corrosion and degradation of magnesium substrates, and enhancing the bone tissue response following implantation. Within the present study, a novel combined approach of anodization and coating (with PEI reinforced with hydroxyapatite nanoparticles) was evaluated, and shown to modulate the corrosion of the Mg alloy, thus controlling Mg release and significantly enhancing bone tissue formation in vivo. The effective control of the alloy degradation and local Mg release may substantiate the improved biological performance of the combined anodized plus coating construct, as within the bone microenvironment the critical range of extracellular Mg concentration is expected to be low, within the 10–20 mM range (Feyerabend et al 2010). Higher Mg levels were found to impair osteoblastic cell function (Serre et al 1998, Feyerabend et al 2010) in a process eventually related to the interference within intracellular calcium oscillations, and with the inactivation of purinergic receptors, resulting in impaired proliferation and differentiation events of differentiating and mature osteoblasts (Zhang et al 2014).
Gene expression in the regenerated bone tissue
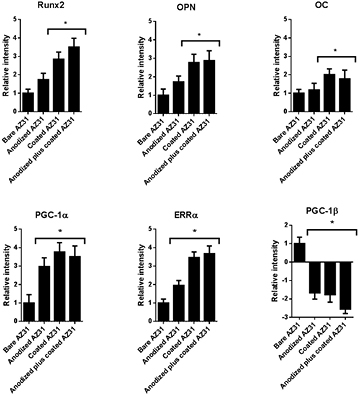
Gene expression analysis, within the bone tissue neighbouring the developed implanted constructs, substantiated the improved osteogenic activity of the coated implants. Data obtained (figure 7) shows a high expression of Runx2, a master regulatory gene for activating osteoblastogenesis, and also of distinct downstream osteogenic markers (i.e. OPNand OC), with the highest levels being achieved in the tissue neighbouring the coated and anodized plus coating samples.
Figure 7. Gene expression data on the newly formed bone tissue ingrowing a defined volume of 0.5 mm towards the implant surface. The mRNA expression levels of Runx2, OPN, OC, PGC-1α, ERRα and PGC-1β genes are relative to GAPDH. Relative mRNA expression is presented in comparison to the baseline levels attained in bare AZ31 samples.
Download figure:
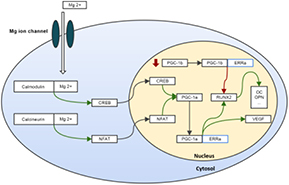
Standard image High-resolution imageAlthough little is known regarding the Mg-mediated transcriptional regulation of the bone tissue formation in vivo, cell culture studies suggest that a controlled Mg release may enhance osteogenic proliferation and differentiation, through specific signalling pathways following cellular uptake by the Mg cell membrane ion channel (Yoshizawa et al 2014a, 2014b). The proposed signalling mechanism is graphically displayed in figure 8, summarizing the key processes discussed.
Figure 8. Schematic representation of the proposed signalling mechanisms ultimately associated with the Mg-mediated enhancement of the osteogenic differentiation. Green arrows represent stimulation and red arrows represent inhibition.
Download figure:
Standard image High-resolution imageOsteogenic activation is expected to occur via PGC-1α signalling, through the activation of members of the calcium signalling pathway (e.g. calmodulin and calcineurin) (Handschin et al 2003), given the high affinity and modulation capability of Mg to these molecules (Ping et al 2004, Grabarek 2011). Calmodulin may modulate PGC-1α expression through a cAMP-response element binding protein transcription factor (Zayzafoon et al 2005), although calcineurin is expected to modulate PGC-1α through NFAT (Zayzafoon 2006), as both have been implicated within the osteogenic-differentiation of precursor populations. The assessment of PGC-1α levels revealed maximal expression within the bone tissue in the vicinity of coated implants (figure 7), thus suggesting that a controlled release of Mg may effectively induce PGC-1α signalling, and upregulate the osteogenic process.
PGC-1α-mediated osteogenic induction has been shown to be dependent on the activation of ERRα (Schreiber et al 2004). In accordance, in the present experiment, ERRα expression correlated well with that of PGC-1α, achieving maximal expression within the tissue in the vicinity of the coated implants (figure 7). The inhibition of ERRα led to the impairment of the osteoblastic differentiation process, although its over-expression enhanced osteogenic commitment, in association with an increased expression of OPNand OC (Bonnelye et al 2001). In fact, ERRα was found to be a transcriptional activator of the master regulator Runx2, given the presence of PGC-1α. Inversely, in its absence, and within the presence of high levels of PGC-1β, ERRα behaves as a negative regulator of Runx2 transcription (Kammerer et al 2013). In the present study, in the bone tissue neighbouring coated implants, high levels of both PGC-1α and ERRα were found, and correlated with a high Runx2 expression, as well as that of its downstream targets, OC and OPN. Simultaneously, a trend for a decreased expression of PGC-1β was verified, further supporting the role of PGC-1α/ERRα mediation in the enhanced osteogenic events associated with the implanted coated constructs. Although this regulatory mechanism has been previously demonstrated within cell culture models, to the best of our knowledge, this is the first in vivo report suggesting the effectiveness of the discussed Mg-induced regulatory pathway within the enhancement of the osteogenic activity.
Summary and conclusion
In this work, distinct surface modification approaches (i.e. anodization and coating with PEI reinforced with hydroxyapatite nanoparticles) were applied on the magnesium alloy AZ31 to modulate corrosion behaviour and to control Mg release following tissue implantation. Tested applications were found to be non-irritating and to lessen the corrosion of the alloy, favouring bone tissue formation within the vicinity of the implant. However, when anodization and coating protocols were combined, the most significant control of corrosion was attained, which induced the most favourable biological response with high levels of vascular ingrowth and bone tissue formation within the vicinity of the implant. The controlled release of Mg is expected to activate PGC-1α/ERRα signalling, that further enhances the expression of relevant osteogenic genes, i.e. Runx2, OPNand OC.
The multifunctional coating proposed in this study for the protection of the Mg alloy AZ31 appears to be a promising strategy to obtain biologically safe and active biodegradable Mg-based implants with prospective applications within bone tissue.
Acknowledgment
The authors acknowledge the financial support under Project ERA MNT/0001/2009.