Abstract
Among the Iron Based Superconductors, the so-called 1144 family attracts significant interest due to its high critical fields and critical current densities and to the stoichiometric nature of the compounds, disentangling the superconducting properties from the compositional homogeneity. Their practical application is partly hindered by the severe and strict synthesis conditions, complicated by high temperature (T > 900 °C) treatments of volatile and toxic elements. In this work, a milder synthetic approach to produce 1144 materials is proposed. A simple one-step High Energy Ball Milling treatment of the pure elements is coupled to a low temperature (i.e. 600 °C) thermal treatment to produce the superconducting Ca/K-1144 material, characterized by a good degree of homogeneity and critical temperatures higher than 30 K. The results here reported demonstrate the previously excluded feasibility of a simple and easily scalable lower temperature synthesis route for a Ca/K-1144 compound. We suggest that the intimate mixing and dispersion of the starting elements promoted by the mechanochemical treatment constitutes a key factor for the successful lowering of the synthesis temperature.
Export citation and abstract BibTeX RIS
1. Introduction
Iron Based Superconductors (IBSCs) attracted in the last years significant attention due to their large critical fields, low anisotropy and high critical currents at very high fields [1]. A lot of experimental efforts have been directed towards the production of wires and tapes, selecting in particular among the different families the 122 compounds (alkaline-earth1 Fe2 As2 [2]) due to their easy synthesis method and grain-boundary tolerance [3]. In these materials, alternate Fe-As layers are intercalated with alkaline-earth metals. Superconductivity, induced by electronic doping either by substituting iron with a divalent metal or by substituting the alkaline-earth metal by an alkaline element, is dependent on the nature of the intercalating elements and on the amount of the dopant. As a close relative of the 122 family, in the last years the 1144 (Alkaline Earth1 Alkaline1 Fe4 As4) family has been discovered [4]. Differently with respect to 122 materials, in the 1144 compounds the Fe-As layers are stacked with alternate planes of either alkali or alkali earth metals, resulting in stoichiometric compounds. The superconducting properties are therefore no more affected by the doping amount (and by in plane random arrangement of alkali-alkali earth). Furthermore, this alternating planes seem to possibly rise to a plethora of defects, resulting in an interesting pinning landscape playground that could greatly enhance critical currents in different field orientations and field-temperature regimes [5].
Several works have been performed on single crystals, grown via the FeAs flux method at temperatures higher than 900 °C [6]. Synthesis of polycrystalline samples have been carried out in similar conditions, with ultra-fast heating methods, high temperature treatments (T > 900 °C) and quench cooling procedures [4, 7–11]. These synthesis methods, despite producing high quality polycrystalline samples, are however not free of drawbacks. For example, the use of the binary compounds as starting materials (e.g. CaAs, KAs, FeAs) requires preceding synthesis steps. Furthermore, high temperature procedures carry in the uncertainty associated with the evaporation of volatile elements and consequently the need to utilize excess amounts of K and As. In addition, ultra-fast heating and cooling procedures often involve a certain degree of uncertainty and scarce reproducibility on large scale. Finally, the safety issues related with the high temperature treatment of elements simultaneously volatile and toxic are not negligible. In this context, the development of lower temperature and easily scalable synthesis process is highly desirable.
It is worth to comment on the general opinion that the 1144 phase cannot be obtained at low temperatures [11]. In particular, it has been reported recently a quite narrow temperature range for the formation of the polycrystalline 1144 phase (e.g. 925 °C–975 °C range in [11]). In the absence of a well assessed phase diagram, the research regarding the actual field of stability of the 1144 phase is however still open. Recent Density Functional Theory (DFT) calculations report that CaK-1144 phase should actually be stable only below a certain critical temperature (i.e. approximately 500 °C [12]), suggesting that its formation at low temperature should not only be possible but favoured with respect to the 122 phases. It is possible to speculate that the observed discrepancy is possible due to kinetic issues and homogeneity of the starting powders, rather than to thermodynamic limits.
In this sense, High Energy Ball Milling (HEBM) could represent a useful tool. HEBM is a cost-effective, low temperature powder processing technique widely applied in material science [13]. Ball milling treatments have already been exploited in the IBSCs synthesis, in particular in powder processing of 122 compounds (e.g. [14]), to promote homogeneity and activate chemical reactions. In fact, during a mechanochemical process, hitting balls transfer high amount of energy to the powder inducing, as the first phenomena to be observed, comminution and intimate mixing of the starting reactant. The milling process can be tailored in several of its variables (material, diameter and hence weight of the balls) which are reflected in the effective energy transferred to the powders. If the energy conditions are met, additional processes can be promoted such as interdiffusion of species, amorphization phenomena or nucleation of new phases. Most importantly, the intimate mixing of freshly formed highly active surfaces can enhance the elemental reactivity, lowering the activation energy of the occurring reactions and thus facilitating the formation of thermodynamically stable chemical compounds at low temperatures [15].
On this basis, a new synthetic approach to produce 1144 samples is proposed in this work. A simple one-step mechanochemical process that utilizes the pure elements as starting materials is coupled to a 600 °C thermal treatment to promote the formation of the 1144 phase. The samples have been characterized by means of x-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) analysis coupled with Energy Dispersive x-ray Spectrometry (EDS) to evaluate microstructure, morphology and chemical composition. Superconducting properties have been evaluated by means of electrical and magnetic measurements. This process leads to high quality materials characterized by a high degree of chemical homogeneity and occurrence of the superconducting transition slightly below 35 K.
2. Experimental
All materials were handled in an Ar glove box (O2 and H2O < 1ppm). Arsenic polycrystalline lumps (Alfa Aesar, 99.99998\,+\,%), Potassium cubes (Alfa Aesar, 99.5%), Calcium granules (Alfa Aesar, 99.5%) and Iron powder (Alfa Aesar, 99.998%) were mixed in an atomic ratio Ca:K:Fe:As = 1.05:1.05:4:4, with a minor excess of the alkaline-earth and alkaline metals to take into account possible oxidation issues. The elements were placed in a 60 ml stainless steel grinding jar with four 12 mm ZrO2 balls in a ball to powder weight ratio of 10:1. The vial was sealed in the glove box and subjected to 2 h of milling in a SPEX 8000 M shaker mill, operating at 30 HZ. Considering a ball velocity for this experimental set-up of 4.2 m s−1 [16], the energy transferred in a single hit by each ball was estimated as Eb = ½ mb vb2 ∼ 27 mJ, with mb and vb mass and ball velocity respectively. By simple calculations, in the reported experiment a total energy Et ∼ 300 kJ g−1 is transferred to the reactants during the process, considering a power Pt = N f Eb ∼ 3.2 W, where N and f are the ball numbers and the hit frequency respectively. Using the milled powder, disk-shaped pellets were prepared by uniaxial pressing and heated in a sealed steel tube with an inner inert crucible similarly as in [17]. The thermal treatment, conducted on several pellets simultaneously, consisted in a heating ramp with a 10 °C min−1 rate up to 600 °C, 10 h of dwell time and a controlled cooling with a 5 °C min−1 rate.
XRD analysis was carried out on the milled powders and on the thermally reacted crushed pellets in sealed glass capillaries using a diffractometer equipped with a 120 ° linear simultaneous detector from INEL and a monochromatized Fe Kα1 source. SEM/EDS analyses were carried out with a Leo 1525 SEM equipped with an Oxford x-ac energy dispersive spectroscopy system. The analysis was carried out on fractured samples obtained directly in the high-vacuum SEM environment by means of a dedicated set-up. Electrical resistance was measured using a standard four contacts method placing the contacts on the edges of the disk-shaped pellets (diameter d≈ 5 mm, thickness h≈ 1 mm). Electrical resistivity was calculated by the van der Pauw method [18]. Onset and offset values were estimated respectively as 90% and 1% of the linear extrapolation of the normal state resistance. Magnetic measurements were performed using of an Oxford Instrument Vibrating Sample Magnetometer (VSM) on the same samples. Measurements were carried out with the field applied perpendicularly to the disk surface, and the results were normalized on the geometrical volume. Direct Current (DC) magnetization was measured in Zero Field Cooling (ZFC) and Field Cooling (FC) conditions applying a 0.002 T magnetic field. For the calculation of the shielded volume, geometrical demagnetization factors were calculated in the disk shape assumption as Nz = 1-πh/d, where h and d are respectively thickness and diameter of the disk [19]. To evaluate granularity phenomena, remanent magnetization (Mrem) was measured as a function of the applied field at the fixed temperature of 5 K as described in [20]. Critical current density was estimated applying the Bean's model [21] from the magnetic hysteresis loops and from remanent magnetization measurements considering a disk shaped sample as Jc= 30 · ΔM/d and Jc= 30 · 2 · Mrem/d, where d is the disk diameter, in the conservative assumption of the current flow occurring through the whole sample.
3. Results and discussion
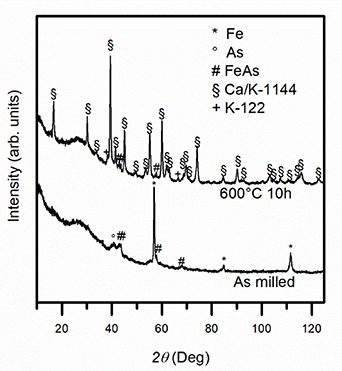
Results of the structural characterization are reported in figure 1. The XRD pattern of the milled powders is characterized by peaks ascribable to mainly unreacted iron and arsenic elements and to an iron arsenide phase, indicating the occurrence of a certain degree of chemical reactions. No feature relative to calcium and potassium compounds can be observed, suggesting that these elements undergo amorphization phenomena during the HEBM treatment.
Figure 1. XRD pattern (FeKα1) of the milled powder and of the heat-treated sample.
Download figure:
Standard image High-resolution imageNo mass loss was observed after the thermal treatment, implying that no evaporation of the volatile elements occurred. The recovered samples show a compact and solid appearance, with geometrical densities of approximately 4.0 ± 0.1 g cm−3. The samples density corresponds to approximately 80% of the theoretical density, estimated to be 5.2 g cm−3 on the basis of the theoretical lattice values [4].
The pattern of the reacted sample powders is reported in figure 1. The Ca/K-1144 (P4/nmm) phase constitutes the majority phase, with minor peaks ascribable to the K-122 phase and to FeAs unreacted residues. A relative estimation of the most intense peaks of these three phases results in an IK122 (103): IFeAs (111): ICaK1144 (103) = 0.06: 0.03: 1 ratio, confirming that most of the sample is formed by the Ca/K-1144 phase. Minor amounts of FeAs and K-122 phases could derive from residues of unreacted reactants, suggesting room for improvement regarding temperature and time of the heat treatment.
Our results demonstrate that the reaction activated by the HEBM treatment proceeds to form the 1144 phase at 600 °C. The obtainment of the 1144 phase at low temperature is in agreement with DFT calculations [12] but constitutes a complete novelty with respect to previous results [4, 8, 11]. We suggest that the high reactivity and uniformity of the elemental powders promoted by the HEBM treatment play a crucial role in this: the mechanochemical treatments is expected to induce the intimate mixing of the elements with high homogeneity degrees on a local scale, and this seems necessary to promote at low temperature the reaction towards the formation of the mixed 1144 phase without the production of separate 122 compounds. It is furthermore important to highlight that the thermal treatment adopted in this work is milder than what has been reported so far, without the need for ultra-fast heating and cooling rates.
The morphology of the heat-treated sample is reported in figure 2. The sample is formed by micrometric aggregates of sub-micrometric particles. As evident in figure 2(a), the morphology is uniform, with few larger and smoother particles (highlighted in the image) dispersed in a nanocrystalline compound. The higher magnification image, reported in figure 2(b), shows the details of the fine morphology of the nanocrystalline structure, characterized by sharp edges and random orientation of the crystallites. Chemical composition, evaluated by EDS measurements, is reported in table 1 for the zones showing different morphology. Regarding the smooth particles, the chemical composition indicates an iron arsenide rich compound (Ca and K ∼ 3 At%, Fe ∼ 40 At% and As ∼ 55 At%), most likely the origin of FeAs peaks in XRD. On the nanocrystalline zones, instead, the overall chemical composition corresponds to the expected CaKFe4As4 phase. Furthermore, it is worth to mention that EDS measurements showed a not negligible diffuse presence of oxygen throughout the fractured section. Considering sample handling and the precautions to avoid environmental contamination, we suppose that oxygen presence is due to an initial contamination of the commercial reactants.
Figure 2. (a) SEM image (secondary electron detector) of the 600 °C heat treated sample, with arrows highlighting smooth Fe-As rich particles; (b) high magnification (in-lens detector) image of the square highlighted in (a) depicting the nanostructured nature of the corrugated regions. The sample pellet has been fractured directly in the high-vacuum SEM environment.
Download figure:
Standard image High-resolution imageTable 1. Summary of EDS results (see text for details). Elemental composition as atomic % ratio (At%) measured in two different zones representative of the sample.
| Ca (At%) | K (At%) | Fe (At%) | As (At%) | |
|---|---|---|---|---|
| Smooth particles | \phantom{1}3 ± 1 | \phantom{1}3 ± 1 | 40 ± 2 | 55 ± 2 |
| Nanocrystalline aggregates | 10 ± 1 | 11 ± 1 | 40 ± 2 | 39 ± 2 |
Regarding the overall composition, in agreement with the absence of mass loss during the thermal treatment, it is observed that the initial composition (Ca:K:Fe:As = 1.05:1.05:4:4) is preserved, and the desired compound is produced without the need of a macroscopic excess of volatile elements reported previously in literature [4]. As an added benefit, such procedure is good at reducing sources of uncertainty and possible inhomogeneity.
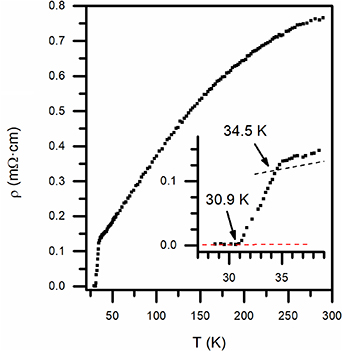
The heat-treated compound was further characterized evaluating electrical and magnetic properties. The resistivity as a function of temperature is reported in figure 3. The resistivity value measured at room temperature, evaluated via the Van der Pauw method, is slightly below 1 mΩ · cm, similar or slightly below previous reports of polycrystalline Ca/K 1144 compounds [4, 11]. The curve exhibits a monotonous decreasing trend, with an approximately linear behaviour below 150 K. An RRR value (ρ290 K/ρ35 K) of approximately 5.5 was calculated from the experimental data. Below 35 K, an abrupt drop in the resistance is observed, evidence of the superconducting transition. Onset and offset values of the transition correspond respectively to 34.5 K and 30.9 K.
Figure 3. Resistivity (ρ) as a function of temperature; in the inset, a zoom of the superconducting transition is shown.
Download figure:
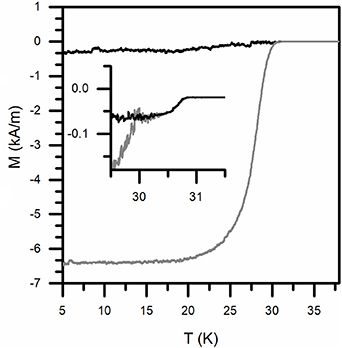
Standard image High-resolution imageThe magnetization M as a function of temperature is reported in figure 4. The sample is characterized by a high diamagnetic response at low temperature, with an almost constant value up to approximately 20 K. The superconducting transition onset, highlighted in the inset, is characterized by an initial decrease of both ZFC and FC curves below 31 K and by a lower temperature splitting of the two curves at approximately 30 K. This curve shape may be due to the presence of several superconducting phases, but considering the structural characterization results and the stoichiometric nature of the Ca/K 1144 superconducting phase, is more likely to ascribe to the insurgence of granularity and grain decoupling phenomena as previously observed in other IBSCs and cuprates [22, 23]. Regarding the shielded volume, susceptibility values close to unity were calculated at 5 K (χ = χexp/(1-Nzχexp) ≈ − 1.0) indicating complete shielding.
Figure 4. Magnetization (M) as a function of temperature for the heat treated sample (ZFC and FC respectively as gray and black curves); in the inset, a zoom of the transition onset is shown.
Download figure:
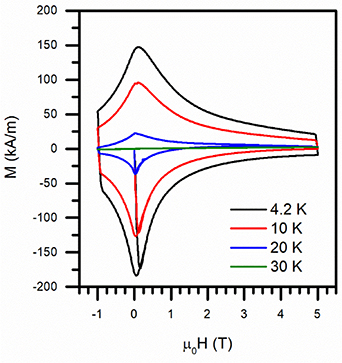
Standard image High-resolution imageMagnetic hysteresis loops measured at different temperatures are reported in figure 5. Below 30 K the sample is characterized by large superconducting hysteresis loops. However, it can be observed a certain degree of asymmetry between the upper and lower branches. Similar behaviour of critical current densities, namely different trends if the field is decreased or increased, has been already observed on IBSCs polycrystalline compounds and can be ascribed once again to granularity issues [9].
Figure 5. Magnetization (M) as a function of the magnetic field measured at different temperatures.
Download figure:
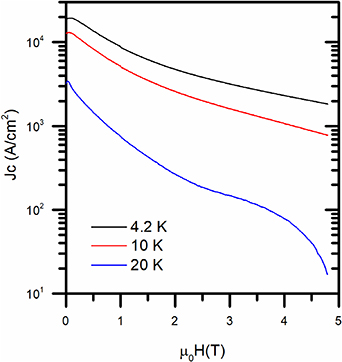
Standard image High-resolution imageCritical current density calculated from the magnetic hysteresis loops is reported in figure 6 as a function of the applied field. The material is characterized by critical current densities values in line with similar polycrystalline samples, with values close to Jc ∼ 2 · 104 A cm−2 at 4.2 K [8, 11]. These results are however orders of magnitude lower than what reported for single crystals of Ca/K 1144 materials, and are characterized by a larger field dependence [5], suggesting once again granularity issues in the polycrystalline material.
Figure 6. Critical current density estimated by means of the Bean's method from the magnetic hysteresis loops at different temperatures.
Download figure:
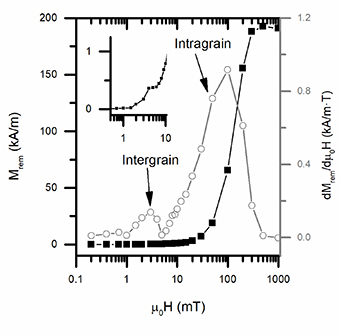
Standard image High-resolution imageTo further evaluate this phenomenon and investigate about possible weak-link phenomena, we performed the measurement of the remanent magnetization Mrem as a function of the applied field as described by Müller et al [20]: in the case of a granular superconductor, magnetic flux will permeate intergranular regions before entering the grains, where it will access only above the lower critical field. The remanent magnetization is therefore expected to show two distinct steps, with magnitude proportional to local current densities flowing in the different regimes. The experimental results are reported in figure 7: as clearly visible in the derivative curve (gray circles), two peaks can be discerned, and associated to intergrain (µ0H∼ 2–3 mT field) and intragrain (µ0H∼ 100 mT field) contribution. Comparing with previous literature regarding 1144 samples, the peak position is comparable, suggesting that similar phenomena exist [11]. A rough estimation performed with the Bean model of the critical current gives a quite low intergrain critical current (Jc < 1 · 102 A cm−2).
Figure 7. Remanent magnetization (Mrem) as a function of the magnetic field (black squares) and its derivative with respect to the field (gray circles) measured at 5 K; in inset, an enlarged view of the Mrem curve in the low field region.
Download figure:
Standard image High-resolution imagePrevious results regarding granularity issues observed in IBSCs [22] and in particular for 122 compounds [24] show that oxygen contaminations tend to accumulate at grain boundaries, with simultaneous segregation of alkaline-earth and alkaline metals. This represent a limitation for the current flow. In this sense, our results could be related to the not negligible oxygen contamination assessed by SEM/EDS measurements. These evidences point out once again the crucial role of the grain boundary stoichiometry for the optimization of the grain connectivity in IBSCs materials, and work is in progress to further tailor the material stoichiometry and remove oxygen contamination sources.
4. Conclusions
In this work, we used a simple one-step mechanochemical treatment coupled to a low-temperature (600 °C) heating step to produce the superconducting Ca/K-1144 compound. Diffraction analysis show that produced material is mainly formed by the desired phase, with only minor traces of the K-122 phase and of a residual FeAs phase. Morphological and chemical analyses show that the sample is formed by sub-micrometric Ca/K-1144 crystals, with high degree of chemical homogeneity. The material is characterized by critical temperature higher than 30 K and wide magnetic hysteresis loops. The magnetic measurements highlight the granular nature of the compound, as commonly observed in similar IBSCs, suggesting that further efforts must be devoted to enhance chemical homogeneity and eventually improve grain boundary connectivity.
The results here reported demonstrate the feasibility of simple and easily scalable low-temperature synthesis routes of Ca/K-1144 compounds, previously excluded in literature. With respect to earlier results, we suppose that the high degree of intimate mixing and dispersion of the pure elements obtained before the thermal treatment through the HEBM process represents a key element for the successful lowering of the synthesis temperature.
Acknowledgments
This work was partially supported by MIUR-PRIN project 'HiBiSCUS'—Grant No. 201785KWLE. The authors wish to acknowledge A Formichetti, F Maierna and L Merli for the precious technical assistance.