Abstract
Exploring high-efficiency and low-cost bifunctional electrodes for supercapacitors and sensors is significant but challenging. Most of the existing electrodes are mostly single-functional materials with simple structure. Herein, NiCo2O4 nanowires as the core and NiMn layered double hydroxide (LDH) as the shell is directly grown in situ on carbon cloth (CC) to form a heterostructure (NiMn LDH@NiCo2O4/CC). The performance in supercapacitors and enzyme-free glucose sensing has been systematically studied. Compared with a single NiCo2O4 nanowire or NiMn LDH nanosheet, the heterogeneous interface produced by the unique core–shell structure has stronger electronic interaction and abundant active surface area, which shows excellent electrochemical performance. Electrochemical tests demonstrate that the NiMn LDH@NiCo2O4/CC core–shell electrode possesses an area specific capacitance of 2.40 F cm−2 and a rate capability of 76.22% at 20 mA cm−2. Simultaneously, asymmetric supercapacitor is assembled with it as the positive electrode and NiFe LDH@NiCo2O4/CC as the negative electrode. The supercapacitor possesses an energy density of 47.74 Wh kg−1 when the power density is 175 W kg−1, revealing excellent performance and maintains cycle stability of 93.48% after 6000 cycles at 10 mA cm−2. Additionally, the electrode applied as enzyme-free glucose sensor electrode also displays outstanding sensitivity of 2139 μA mM−1 cm−2, wide detection range (2 μM−3 mM and 4–8 mM) and low detection limit of 210 nM, representing good anti-interference performance. This work reveals the multi-metal synergy and rationally designed core–shell structure is critical to the electrochemical performance of bifunctional electrodes.
Export citation and abstract BibTeX RIS
1. Introduction
Due to the increasing scarcity of fossil energy and serious environmental pollution caused by combustion emissions, low-carbon economic development model has been advocated, and the development of renewable energy is particularly urgent [1–3]. As one of the renewable energy storage devices, supercapacitor has received extensive attention due to it remarkable advantages such as long service life, high charging and discharging efficiency and excellent power density [4, 5]. At present, cathode materials with the reasonable structure and electrochemical performance are significant to enhance the storage capacity of supercapacitors [6, 7]. The reversible redox reaction of pseudo-capacitor materials occurs on the surface of the electrode or even in the whole electrode to store energy, therefore it can obtain higher capacitance and energy density than a double-layer capacitor [8–11]. As typical pseudo-capacitor materials, transition metal oxides have always been the focus of research in the field of supercapacitors. In addition to energy storage, pseudo-capacitor materials also exhibit superior electrocatalytic properties for sensors [12–14]. However, the application conditions of enzyme biosensors are harsh, which cannot guarantee the detecting life and stability [15]. Electron-mediated sensors have obtained widespread notice owing to high sensitivity, fast response, long life and good anti-interference [16–18]. Based on the outstanding performance of pseudo-capacitor materials in different electrochemical fields, the exploration of multifunctional electrode materials with high electrochemical activity presents a broad prospect in the future [19–21].
NiCo2O4 as a typical transition metal oxide with spinel structure is used in energy storage, catalysis, sensing and other fields owing to its abundant resources and low price [22–26]. Compared with single component NiO and Co3O4, NiCo2O4 presents better performance due to the synergistic effect of bimetallic [27]. However, single structure electrodes such as NiCo2O4 cannot meet the current demand for electrochemical performance, so the controlling of composition and structure for electrode materials become important. It is reported that NiMoO4@CoMoO4 core–shell arrays grown on nickel foam (NF) achieved a good specific capacitance of 5.4 F cm−2 at 2 mA cm−2, the specific capacitance still maintained 3.1 F cm−2 at 40 mA cm−2 [28]. CuCo2S4@NiMn-LDH core–shell array by hydrothermal in situ synthesis on nickel foam, the specific capacitance is 2520 F g−1 at the current of 2 A g−1, which has excellent rate performance and cycle life [29]. The flower-shaped CuCo2O4 nanosheets with graphite paper as the conductive substrate have obtained good sensitivity (3.625 μA μM−1 cm−2) and detection limit (5 μM) when applied to the enzyme-free glucose sensor [30]. ZIF-67 grew on the prepared NiCo2O4 nanowires, and annealed to obtain Co3O4/NiCo2O4/CC composites, it showed wide linear range (1 μM–1.127 mM) with good sensitivity (12.835 mA mM−1 cm−2), low detection limit (0.64 μM) [31].
On the other hand, layered double hydroxide (LDH) has been applied in supercapacitors and sensors because of high theoretical capacity, simple preparation, low price and easy availability [32, 33]. It is reported that the doping of Mn ions can significantly enhance the electrochemical activity of Ni ions in LDHs [34]. However, the agglomeration of NiMn LDH is inevitable in the growth process, which cannot effectively transfer electrons, resulting in the accumulation of a large number of electrons, the decrease of intrinsic conductivity and the serious limitation of electrochemical capacity. Therefore, the combination of NiMn LDH with nanowires can effectively reduce the agglomeration of NiMn LDH. It is confirmed that core–shell nanomaterials based on nanowires and nanosheets demonstrate excellent electrochemical properties [35, 36]. Currently, there are few studies on the bifunctional performance of electrode materials based on NiMn LDH@NiCo2O4 core–shell structure in supercapacitors and glucose sensing, therefore, it is investigated depthly in this field.
In this work, the three-dimensional self-supporting core–shell structure electrode (NiMn LDH@NiCo2O4/CC) was successfully prepared by hydrothermal and annealing methods. Carbon cloth as a conductive substrate shows superior conductivity, and NiCo2O4 nanowires as a core structure provide stable electron transmission channels. The active center of the material can be effectively exposed based on the rich specific surface area of the NiMn LDH shell. As a bifunctional material, the electrode has shown superior performance when applied to supercapacitors and glucose sensor. It possesses high area specific capacitance and outstanding rate capability. At the same time, an asymmetric supercapacitor (ASC) was constructed with NiFe LDH@NiCo2O4/CC as the negative electrode and NiMn LDH@NiCo2O4/CC as the positive electrode. The ASC shows excellent energy density and cycle life. Moreover, as an electrode for glucose sensor, the NiMn LDH@NiCo2O4/CC shows wide detection range with high sensitivity, low detection limit and good selectivity. The electrode also has impressive anti-interference performance with ascorbic acid, uric acid, dopamine and other interference substances. It provides a new idea for the research of bifunctional materials in the future.
2. Experimental methods
2.1. Materials
Cobalt nitrate hexahydrate [Co(NO3)2·6H2O], Nickelnitrate hexahydrate [Ni(NO3)2·6H2O], Urea [CO(NH)2], Nickel chloride hexahydrate [NiCl2·6H2O], Manganese Chloride Tetrahydrate [MnCl2·4H2O], Hexamethylenetetramine [C6H12N4, HMT], Iron (Ⅲ) nitrate nonahydrate [Fe(NO3)3·9H2O], Carbon cloth (CC). All chemicals used above were of analytical grade.
2.2. Preparation of NiCo2O4/CC
The NiCo2O4/CC was prepared by hydrothermal method, 1 mmol Ni(NO3)2·6H2O, 2 mmol Co(NO3)2·6H2O and 12 mmol CO(NH2)2 were dissolved in 80 ml deionized (DI) water by stirring, then the mixture and a piece of CC were transferred to a 100 ml Teflon-lined stainless autoclave, which heated at 120 °C for 6 h. Then the NiCo2O4/CC was taken out, rinsed with DI water for several times and heated at a temperature rise rate of 5 °C min−1 in N2 flow (350 °C for 2 h).
2.3. Preparation of NiMn LDH@NiCo2O4/CC
In a typical synthesis, 3 mmol NiCl2·6H2O, 1 mmol MnCl2·4H2O and 5 mmol HMT were dissolved in 70 ml DI water by stirring. The as-obtained NiCo2O4/CC arrays with the solution above were transferred to a 100 ml Teflon-lined stainless autoclave and heated at 90 °C for 8 h. The product was collected and rinsed with DI water, then dried at 60 °C overnight. The estimated loadings of NiMn LDH@NiCo2O4 on carbon cloth is 0.98 mg cm−2.
2.4. Preparation of NiFe LDH@NiCo2O4/CC
3 mmol Ni(NO3)2·6H2O, 1 mmol Fe(NO3)3·9H2O and 20 mmol CO(NH2)2 were dissolved in 50 ml DI water and stirred, then transferred into an autoclave with the NiCo2O4/CC and heated at 120 °C for 8 h. Finally, the product was rinsed with DI, dried at 60 °C overnight. The estimated loadings of NiFe LDH@NiCo2O4 on carbon cloth is 1.02 mg cm−2.
2.5. Electrochemical measurements
Electrochemical testing for supercapacitors: the electrochemical performance was studied using the Electrochemical Workstation (CHI 760E, Chen Hua, Shanghai). In a three-electrode system, the prepared material was employed as the working electrode, and the standard mercury/mercury oxide (Hg/HgO) and platinum electrode were used as the reference and counter electrode, respectively. The electrolyte used for galvanostatic charge–discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) analysis was 2 M KOH. For the frequency range of EIS was 0.01–100 kHz with the amplitude potential of 0.005 V, and the measured potential was consistent with the open circuit voltage.
The specific capacitance can be calculated by the formula:
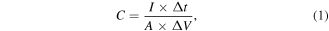
where I and Δt is the current (mA) and time (s) during discharge process, respectively, A is active area of electrode (1 cm2), and ΔV is voltage range. When A is replaced by m (the mass of active material), the gravimetric capacitance is obtained.
In ASCs, the loading capacity of the active material on the electrodes can be calculated by the following:
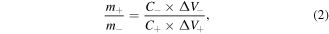
where m+ and m−, ΔV+ and ΔV−, C+ and C− represent the mass of active substance loaded by positive and negative electrodes, potential ranges and specific capacitances, respectively. The load of the positive electrode was 0.98 mg before assembling. According to formula (2), the calculated theoretical load of the negative electrode should be 2.72 mg, and the actual load was 2.68 mg for assembling to achieve the charge balance.
The formulas for calculating energy density and power density are as follows:
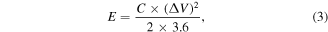
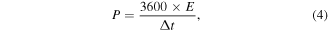
where C is specific capacitance of ASCs, ΔV (V) and Δt (s) are the voltage ranges and discharge time, respectively.
Electrochemical test for glucose sensor: All related electrochemical tests for glucose sensor (CV and i–t) were performed in the three-electrode system using the same electrodes as above, except that the electrolyte concentration was changed to 1.5 M KOH.
2.6. Characterization
The structure and morphology characterization of the electrodes were analyzed by field-emission scanning electron microscope (FESEM, Hitachi S4800, Japan) and transmission electron microscope (TEM, Hitachi H7650, Japan). The x-ray diffraction (XRD, Bruker D8 Discover) measurements with a Cu–Kα radiation from 5° to 80° (2θ range), x-ray photoelectron spectroscopy (XPS, Axis Ultra HAS with Al K radiation) were used to study the crystal structure and composition of all electrodes.
3. Results and discussion
3.1. Characterization of NiMn LDH@NiCo2O4/CC electrode
Schematic diagram for the fabrication process of NiMn LDH@NiCo2O4/CC electrode is shown in figure 1. Firstly, NiCo LDH nanowires (NWs) are grown directly on a carbonized hydrophilic carbon cloth (CC) by hydrothermal treatment in deionized water containing Ni2+, Co2+ and CO(NH)2. CC as a conductive substrate provides support for the in situ growth of NiCo LDH NWs and greatly improves the conductivity of electrodes. Secondly, the roughness of the NiCo LDH NWs increases after further calcination. Finally, the NiMn LDH@NiCo2O4/CC electrode is fabricated by hydrothermally loading NiMn LDH nanosheets on NiCo2O4 NWs. The unique core–shell structure of the electrode provides an abundant surface area for the redox reaction and exposes more active sites, which enables the active substances on the electrode fully contact the electrolyte.
Figure 1. Schematic illustration of the NiMn LDH@NiCo2O4/CC electrode.
Download figure:
Standard image High-resolution imageAs shown in figures 2(a), (b), NiCo2O4 grow uniformly and densely on carbon cloth fibers, and the length of the nanowires present approximately 2 μm and the diameter is 40 nm. This preparation method leaves many gaps and provides space for the subsequent growth of NiMn LDH. In figures 2(c), (d), NiMn LDH is directly synthesized on CC by hydrothermal method for comparative electrode. The NiMn LDH nanostructures are honeycombed and completely wrapped on the carbon fibers. Figures 2(e), (f) displays the morphology of NiMn LDH@NiCo2O4/CC at different magnifications, the NiMn LDH nanosheets are grown uniformly on the NiCo2O4 NWs. In addition, the nanosheets are cross-connected to build a 3D network structure with a high open space. In this way, the core–shell structure with rich surface area can provide abundant channels for chemical reactions, achieve sufficient contact between electrodes and electrolytes, and facilitate the transfer of ions and electrons. Figures 2(g), (k) shows the SEM and the EDS images of the NiMn LDH@NiCo2O4/CC. The existence and the uniform distribution of Ni, Co, Mn and O elements in the core–shell structure are further confirmed. Figures S1(a), (b) (available online at stacks.iop.org/NANO/32/505710/mmedia) also displays two-dimensional sheets of NiMn LDH shell structures are distributed around NiCo2O4 NWs, which indicates the successful preparation of electrode materials.
Figure 2. SEM images of (a)–(b) NiCo2O4/CC, (c)–(d) NiMn LDH/CC and (e)–(f) NiMn LDH@NiCo2O4/CC at different magnifications, (g)–(k) SEM image and the corresponding EDS elemental mapping of Ni, Co, Mn, and O for NiMn LDH@NiCo2O4/CC.
Download figure:
Standard image High-resolution imageAs shown in the figure 3(a), the accurate crystal phase and crystalline structure of the prepared NiMn LDH@NiCo2O4/CC electrode were characterized by XRD. According to the analysis of the JCPDS No. 73-1702 card and NiCo2O4/CC peaks, it is clearly shown that there are obvious characteristic diffraction peaks (denoted by '♥') at 36.8°, 44.5° and 62.8°, corresponding to the (311), (400) and (440) planes of NiCo2O4 phases [37], respectively. Furthermore, the peaks (denoted by '♦') at 11.5°, 22.7°, 34.6°, 59.9° are indexed to (003), (006), (012) and (110) planes of the NiMn LDH (JCPDS No. 38-0715) [38, 39]. Finally, the above specific diffraction peaks of NiMn LDH@NiCo2O4/CC are all shown in the XRD curves, indicating that the electrodes with NiCo2O4 as core and NiMn LDH as shell are prepared successfully. It is noteworthy that the NiMn LDH peak in the XRD pattern is wider and has a lower intensity because of the poor crystallinity of LDH and the less NiMn LDH nanosheets grown on the carbon cloth. To further comprehend the chemical state of the electrode, XPS was used for quantitative and qualitative analysis of the sample, as shown in the figure 3(b). The full scan spectrum reveals that the electrode contained Ni, Co, Mn, O, C and N elements. In figure 3(c), the spectrum of Ni 2p displays two satellite peaks considered as 'sat', in company with two spin–orbit peaks at 873.2 (Ni 2p1/2) and 855.5 eV (Ni 2p3/2) [40], both of which can be split into two sub-peaks corresponding to the Ni2+ and Ni3+ oxidation states, respectively [41]. Figure 3(d) further illustrates the spectrum of Co 2p, in which the two peaks located at 796.7 (Co 2p1/2) and 781.2 eV (Co 2p3/2) are originated from the presence of Co2+ and Co3+ [42]. As shown in figure 3(e), two satellites appear at 654.1 and 643.3 eV, which are corresponded to Mn 2p1/2 and Mn 2p3/2 of the trivalent Mn3+ oxidation state of NiMn LDH [43]. For the O1s XPS spectrum shown in figure 3(f), the three peaks at 532.5 (O3), 531.3 (O2) and 530.7 (O1) eV are assigned to typical metallic oxygen, hypoxic defects and surface adsorption H2O defects, respectively [43]. In summary, the conclusions obtained from the XPS spectrum are consistent with the results of XRD and SEM, demonstrating that the NiMn LDH@NiCo2O4/CC electrode material with core–shell structure has been successfully synthesized.
Figure 3. (a) XRD pattern, (b) the full XPS spectrum, and high-resolution XPS spectrum of (c) Ni 2p, (d) Co 2p, (e) Mn 2p, (f) O 1 s of NiMn LDH@NiCo2O4/CC.
Download figure:
Standard image High-resolution image3.2. Electrochemical behavior towards supercapacitor
Electrochemical tests were carried out by further evaluating the electrodes. Figure 4(a) shows the integral area of the NiMn LDH@NiCo2O4/CC electrode presents the largest at 10 mV s−1, revealing the specific capacitance is the highest among three electrodes. Moreover, the oxidation and reduction peaks of the electrode is obvious and symmetrical, which suggests that the NiMn LDH@NiCo2O4/CC electrode possesses a higher electrochemical activity and outstanding energy storage capability. Figure 4(b) displays the GCD curves of NiCo2O4/CC, NiMn LDH/CC and NiMn LDH@NiCo2O4/CC at 5 mA cm−2. The discharge time of them are 35, 120 and 270 s, respectively, illustrating the NiMn LDH@NiCo2O4/CC electrode presents a higher specific capacitance [10]. Figure 4(c) displays the CV curves of the NiMn LDH@NiCo2O4/CC electrode at different scan rates (5, 10, 30, 50, 70 and 80 mV s−1). Obviously, all CV curves have roughly the same trend and symmetric redox peaks, demonstrating that the capacitance is derived from a rapid Faraday reaction [44]. The anode peak and the cathode peak move in the positive and negative directions at various scan rates owing to the better reversibility of the electrode, and which is also influenced by the increase of internal diffusion resistance and slight polarization phenomena. Figure 4(d) illustrates that the GCD curves of the NiMn LDH@NiCo2O4/CC electrode at different current densities (1–20 mA cm−2) under voltage window of 0–0.5 V. Each curve possesses an obvious platform, and the charge and discharge time is also identical. It is indicated that the electrode shows high redox reaction reversibility and Coulomb efficiency. The corresponding area specific capacitance is calculated to be 3.09, 2.98, 2.71, 2.56, and 2.40 F cm−2 at 1, 2, 5, 10 and 20 mA cm−2 (3153, 1520, 553, 261 and 122 F g−1 at 1, 2, 5, 10 and 20 A g−1). The specific capacitance of the electrode decreases with the enhancement of current density because of insufficient chemical reaction under high current. As shown in figure 4(e), the optimized electrode still maintains outstanding specific capacitance of 76.22% from 1 to 20 mA cm−2. It can be observed that the capacitance performance of the NiMn LDH@NiCo2O4/CC electrode is significantly superior than that of NiCo2O4/CC and NiMn LDH/CC, which is attributed to the distinctive core–shell structure. The surface roughness of calcined NiCo2O4 NWs increases, and which assists NiMn LDH nanosheets in growing more oriented and firmly on the nanowires without aggregation. The combination of different compositions and structures can produce synergistic effects and increase functionality. Therefore, the core–shell structure behaves better than unitary structure [45].
Figure 4. Comparison of NiCo2O4/CC, NiMn LDH/CC and NiMn LDH@NiCo2O4/CC: (a) CV curves of the electrodes at 10 mV s−1, (b) GCD curves of the electrodes at a charge current density of 5 mA cm−2, (c) CV curves of the NiMn LDH@NiCo2O4/CC electrode at different scan rates, (d) GCD curves of the NiMn LDH@NiCo2O4/CC electrode at a charge current density of 1–20 mA cm−2, (e) capacitance retentions of the NiMn LDH@NiCo2O4/CC electrode, (f) Nyquist plots.
Download figure:
Standard image High-resolution imageTo further explain the ion diffusion kinetics of the NiMn LDH@NiCo2O4/CC electrode, the EIS test was performed, as shown in figure 4(f). The EIS curve is mainly divided into two parts [46]: the high frequency area and the low frequency area represented by a semicircle and the diagonal line. The slope of the curve in low frequency region represented by NiMn LDH@NiCo2O4/CC is the highest, revealing the smallest ion diffusion resistance (Rw) and the strongest diffusion ability, reflecting OH− can transport and exchange electrons freely near the interface of the electrode [47]. The response of the high frequency area of EIS can be evaluated from the partial enlarged graph of the curve. It is obvious that the intercept between the curve and the real axis is very small, which means that the interface resistance (Rs) between the electrolyte and the electrode is low, illustrating that the core–shell structure is beneficial to the transport of ions and increase the active area of chemical reactions. The NiMn LDH@NiCo2O4/CC electrode maintains outstanding capacitance retention and low resistance owing to the superior electrical conductivity of carbon cloth substrate and the synergistic behavior of the core–shell structure.
Generally, the CV curve of the electrode reflects the capacitance behavior and the diffusion control process. In order to further clarify the charge storage mechanism of the electrodes, the CV tests are used to calculate the relationship between different scan rates and peak current shown in formula (5) [48], which can be applied to analyze the capacitance behavior of the electrodes during the charge and discharge processes
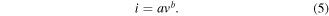
As shown in the formula (5), i (mA) and v (mV s−1) mean the different peak current values and scan rates, respectively. Where a and b are both adjustable coefficients. The value of slope b is obtained by fitting the straight line according to the above formula. The range of b value is mainly divided into 0.5, 0.5–1 and ≥1, which represents the battery property, coexist battery and pseudo-capacitance properties, and pseudo-capacitance property, respectively. Figure 5(a) shows that the electrode possesses dual properties of battery and pseudo-capacitance. It also means the electrode presents both diffusion and capacitance control during charging and discharging [49].
Figure 5. (a) Logarithmic plot of anode peak current and scan rate, (b) the contribution ratio of different electrodes to capacitive-controlled processes at the same scan rates (5, 10, 30, 50, 70 and 80 mV s−1), Capacitance contribution rate of electrodes at a scan rate of 5 mV s−1: (c) NiCo2O4/CC, (d) NiMn LDH/CC, (e) NiMn LDH@NiCo2O4/CC.
Download figure:
Standard image High-resolution imageAccording to formula (5), the contribution rate of pseudo-capacitance to charge storage is calculated as follow:
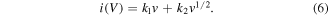
In this formula, i (V, mA) represents the current value corresponding to a specific voltage at various scan rates (v, mV s−1), while k1 and k2 are adjusted parameters. For the current value corresponding to each voltage, i(V)/v1/2 and v1/2 in formula (6) can be fitted to calculate the corresponding value of k1 . Figure 5(b) depicts the contribution curves of the pseudo-capacitance of the three electrodes, and which are calculated from a number of k1 v values. Obviously, the capacitance contribution enhances as the scan rate increases because the diffusion-controlled current presents a slower time response. The transfer and storage of charges on the electrode surface is easier to proceed with the scan rate increases due to the surface adsorption. As shown in figures 5(c)–(e), the contribution to area specific capacitance of the NiMn LDH@NiCo2O4/CC (32.88%) electrode is larger than NiMn LDH/CC (23.67%) and NiCo2O4/CC (19.71%) at 5 mV s−1, confirming the superiority of the NiMn LDH@NiCo2O4 core–shell structure. The analysis of the capacitance contribution rate verifies that the core–shell structure of 2D nanosheets uniformly grown on 1D nanowires can effectively increase the specific surface area, which provide abundant active centers for the reaction and improve the electron transport efficiency.
To evaluate the electrochemical performance of the NiMn LDH@NiCo2O4/CC electrode, an ASC with NiMn LDH@NiCo2O4/CC as the positive electrode and NiFe LDH@NiCo2O4/CC as the negative electrode were assembled. As shown in figure 6(a), the CV curve of NiFe LDH@NiCo2O4/CC is similar to a rectangular structure compared with that of NiMn LDH@NiCo2O4/CC, which means that the negative electrode possesses the performance of electric double-layer capacitance. And the GCD profile of the NiFe LDH@NiCo2O4/CC electrode is shown in figure S2. The gravimetric capacitance of NiFe LDH@NiCo2O4/CC electrode is calculated, which are 812, 378, 146, 70 and 34 F g−1 at 1, 2, 5, 10 and 20 A g−1. In figure 6(b), the assembled ASC is tested by CV curves under different voltages to determine the broadened voltage window at 10 mV s−1. Polarization of CV curve could be observed when the voltage is greater than 1.4 V, therefore the voltage window is selected to 0–1.4 V. Figure 6(c) displays the CV tests of ASC at 10–100 mV s−1, and the shape does not change a lot as the scan rate increases, revealing the outstanding fast charging and discharging performance of ASC. In figure 6(d), it shows that the GCD curves of the equilateral triangle shape, demonstrating that ASC presents typical double-layer capacitor characteristics. The gravimetric capacitance of ASC are 174, 167, 152, 147, 100, and 89 F g−1 at 0.5, 1, 1.5, 2, 4, and 5 A g−1. When the current is increased 10 times from 0.5 A g−1 to 5 A g−1, it can still maintain a rate capability of 51.15% (figure S3). As can be seen in figure 6(e), the power and energy density are respectively calculated. It shows that the energy density of the electrode reaches the maximum of 47.74 Wh kg−1 at the power density of 175 W kg−1, which is more excellent than other supercapacitors [29, 50–53], confirming that the ASC shows a broad practical application prospect. Figure 6(f) shows the capacitance retention rate can reach 93.48% after 6000 cycles at 10 mA cm−2, which displays better cycle stability. As shown in Video 1, the ASC can drive a small fan to rotate after charging, indicating the prepared NiMn LDH@NiCo2O4/CC electrode shows application potential for the future research of energy storage devices.
Figure 6. (a) CV curves of the NiMn LDH@NiCo2O4/CC and NiFe LDH@NiCo2O4/CC electrodes at 10 mV s−1, (b) CV curves of NiMn LDH@NiCo2O4/CC//NiFe LDH@NiCo2O4/CC ASC in different voltage ranges measured at 10 mV s−1, (c) CV curves of the device at different scan rates, (d) charge–discharge curves at various current densities, (e) ragone plot, (f) cycling stability of the device at 10 mA cm−2 (inset shows the first and last GCD cycles).
Download figure:
Standard image High-resolution image3.3. Electrochemical behavior towards glucose sensor
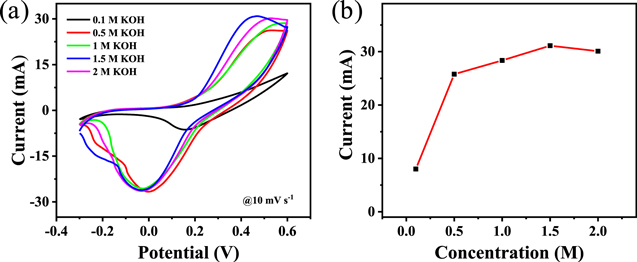
The electrolyte concentration shows an crucial influence on the performance of different electrodes [54]. Figures 7(a), (b) displays the CV measurements of NiMn LDH@NiCo2O4/CC with different concentrations of KOH solution (adding 1 mM glucose) at 10 mV s−1. The current response of the electrode to 1 mM glucose increases with the gradual increase of the electrolyte concentration (0.5–1.5 M). The current response is the largest when the electrolyte concentration is 1.5 M, indicating that the appropriate OH− concentration is beneficial to improve the catalytic efficiency and promote the occurrence of redox reactions. However, the current response of the electrode to 1 mM glucose decreases when the electrolyte concentration reaches 2 M, revealing that the high concentration of OH− would hinder the catalytic activity of the electrode to glucose. Therefore, the optimum concentration of KOH is 1.5 M for glucose oxidation.
Figure 7. (a) CV curves of the NiMn LDH@NiCo2O4/CC electrode in different concentrations KOH solution with 1 mM glucose at 10 mV s−1, (b) the corresponding plot of the anodic peak current density and KOH concentration.
Download figure:
Standard image High-resolution imageFigures 8(a), (b) displays that the CV curves of the electrode with 1 mM glucose in 1.5 M KOH. It is clearly seen that the current peaks show a regular positive shift with increasing scan rate (5–70 mV s−1). From the linear fitting results of peak current, the surface control process affects the electrochemical reaction [55]. Figure 8(c) displays the CV curves of different electrodes in the absence and the presence of 1 mM glucose in 1.5 M KOH. It is observed that the NiMn LDH@NiCo2O4/CC exhibits a superior current density and integral area than other electrodes. The current density of the anode peak increases due to the presence of glucose, illustrating that the electrode is beneficial to the oxidation of glucose. To further prove the electrochemical response of electrodes to glucose, it is tested with 0–8 mM glucose added in 1.5 M KOH. Figure 8(d) shows the peak current of the anode increases regularly with the enhancement of glucose concentration and the correlation diagramas is shown in figure S4, showing that the NiMn LDH@NiCo2O4/CC electrode has good electrochemical activity to glucose. The unique core–shell structure provides more active area and facilates the glucose oxidation [56, 57], and the reactions are summarized by the following chemical equations (M = Ni, Co, Mn):
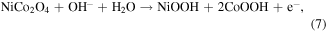
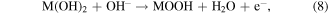
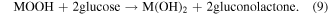
Figure 8. (a) CV curves of the NiMn LDH@NiCo2O4/CC electrode with 1 mM glucose in 1.5 M KOH at various scan rates, (b) the linear relationship between of current density of scan rate and anode and cathode peak, respectively, (c) CV curves for different electrodes with or without glucose in 1.5 M KOH at 10 mV s−1, (d) the CV curves of the NiMn LDH@NiCo2O4/CC electrode after the addition of different concentrations of glucose in 1.5 M KOH at 10 mV s−1.
Download figure:
Standard image High-resolution imageThe sensitivity displays a vital standard to judge the performance of sensors. As depicted in figures 9(a), (b), the optimal working voltage of the electrode is selected through i–t test and linear fitting. It can be observed that the current response of the NiMn LDH@NiCo2O4/CC electrode increases with the enhancement of the working voltage. When the voltage is +0.45 V, there is a relatively high current response and a stable baseline, and the degree of fit (R2) of the linear fit is large. Therefore, +0.45 V is selected as the optimal working voltage. Figure 9(c) shows the i–t response of different electrodes to 1 mM glucose solution. Obviously, the NiMn LDH@NiCo2O4/CC electrode displays the highest current response toward glucose. Figure 9(d) displays the linear fitting relationship between glucose concentration and time for different electrodes. The NiMn LDH@NiCo2O4/CC electrode has a better fitting. The results indicate that the electrode shows outstanding sensing performance, which is attributed to the core–shell structure exposing more active sites and promoting the occurrence of catalytic reactions.
Figure 9. (a) Amperometric i–t response of the NiMn LDH@NiCo2O4/CC electrode after adding 0.1 mM glucose continuously to 1.5 M KOH under different voltages, (b) the linear fitting relationship between glucose concentration and time on the NiMn LDH@NiCo2O4/CC electrode at various voltages, (c) amperometric i–t response of different electrodes after adding 0.1 mM glucose continuously to 1.5 M KOH under +0.45 V, (d) the linear fitting relationship between glucose concentration and time on different electrodes.
Download figure:
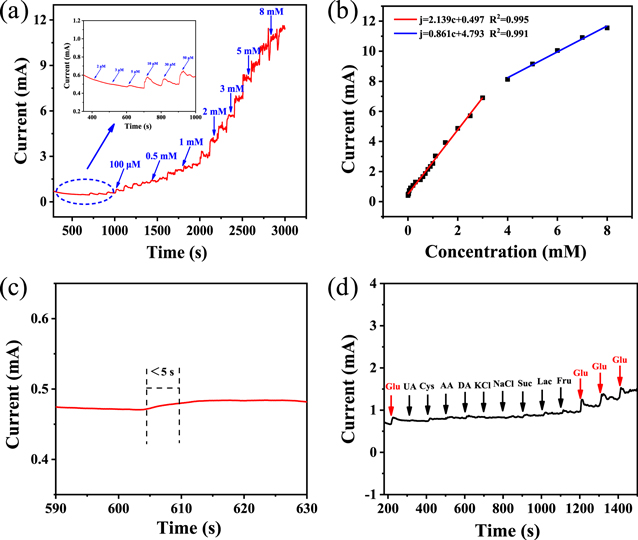
Standard image High-resolution imageFigure 10(a) shows the i–t curve of continuously adding of glucose with different concentrations to 1.5 M KOH solution within 3000 s. It can be seen the current density begin to respond significantly when the glucose concentration approaches 2 μM from the partial enlarged detail. The current response is stable and slow at low concentrations, while the response becomes faster at higher glucose concentrations. However, when the concentration is close to 8 mM, the baseline fluctuates greatly and the linear trend begins to deviate, indicating that the glucose concentration is high. As shown in figure 10(b), the electrode shows different linear correlation in the range of 2 μM−3 mM and 4–8 mM with sensitivities of 2139 and 861 μA mM−1 cm−2. And the minimum detection limit can be calculated as follow formula: LOD = 3Sb/Kl (S/N = 3). Figure 10(c) shows that the current responds quickly within 5 s. At the same time, the anti-interference performance of the electrode is evaluated. The concentration of each interference species (UA, Cys, AA, DA, KCl, NaCl, Suc, Lac, Fru) is 0.1 mM. In the anti-interference test process, glucose and interfering substances are added to the electrolyte sequentially [58, 59]. Figure 10(d) displays the current response is rapidly amplified when 0.1 mM glucose is added in 1.5 M KOH. However, the current density presents no response or minimal response when other interferents are added, and the current density rises rapidly when glucose is added again, revealing that the electrode behaves good anti-interference performance. In conclusion, the electrode shows higher sensitivity, lower detection limit and better anti-interference ability, which provides a new strategy for the research of glucose sensor in the future.
Figure 10. (a) Amperometric i–t response of the NiMn LDH@NiCo2O4/CC electrode with different glucose concentrations in 1.5 M KOH under +0.45 V, (b) the linear fitting relationship between glucose concentration and time on the electrode under +0.45 V, (c) the reaction time of the electrode after the addition of glucose, (d) the anti-interference performance curve of the electrode for glucose sensor.
Download figure:
Standard image High-resolution imageThe reproducibility of the NiMn LDH@NiCo2O4/CC electrodes were evaluated by six fabricated electrodes, as shown in figure 11(a). The relative standard deviation (RSD) is calculated as 2.49%. In figure S5, the sensitivities of six different electrodes (0.1 mM glucose is added every 100 s) are tested, and the RSD value is only 3.95%, indicating that the electrode has good reproducibility. In order to measure the long-time stability of the electrodes, the oxidation peak current of the electrode with 1 mM glucose is measured every 5 d at 35 mV s−1. Figure 11(b) shows the NiMn LDH@NiCo2O4/CC electrode can still maintain a current density of 89.87% after 30 d, demonstrating that the electrode material has superior reproducibility and stability. To evaluate the mechanical stability of the core–shell heterostructure, the SEM images of the electrode after glucose sensing are collected. As shown in figures S6(a), (b), the fibers on the surface of the electrode after glucose sensing are intact without breaking, and the morphology of the core–shell heterostructure has not collapsed. In addition, the CV curves have no change significantly before and after the electrode is bent (figures S6(c), (d)), revealing that it has good mechanical stability. In general, compared with other similar electrodes in table 1, the NiMn LDH@NiCo2O4/CC electrode exhibits higher sensitivity, larger detection range and minimum detection limit towards glucose oxidation. The results show that the core–shell structure electrode represents outstanding advantages when applied to glucose sensors.
Figure 11. (a) Reproducibility test of the NiMn LDH@NiCo2O4/CC electrodes in 1.5 M KOH containing 1.0 mM glucose for six different electrodes, (b) stability of the NiMn LDH@NiCo2O4/CC electrode stored at room temperature for 30 d.
Download figure:
Standard image High-resolution imageTable 1. Comparison of the performance of the prepared glucose sensor with other reported non-enzymatic glucose sensors.
| Electrode | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | LOD (μM) | References |
|---|---|---|---|---|
| NiCo2O4 NWs/CC | 4120 | 0.001–0.63 | 0.5 | [58] |
| core–shell IrO2@NiO NWs/GCE | 1439.4 | 0.0005–2.5 | 0.31 | [59] |
| NiFe2O4/Carbon | 24.6 | 3.2–12.4 | 98 | [60] |
| NiFe2O4-NiCo-LDH@rGO | 111.86 | 0.035–4.525 | 12.94 | [61] |
| Ni3S2/MWCNT-NC | 3345 | 0.03–0.5 | 1 | [62] |
| NiCo2O4/ECF | 1947 | 0.005–19.175 | 1.5 | [63] |
| Co-CoO-Co3O4 | 949 | 0.002–6.06 | 0.58 | [64] |
| NiMn LDH@NiCo2O4/CC | 2139 | 0.002–3 | 0.21 | This work |
| 861 | 4–8 |
The core–shell heterostructure can improve the charge transfer ability of the redox reaction, and the synergistic effect of the multi-element metal is beneficial to improve the conductivity when it is used as the electrode of the supercapacitor [65]. In addition, since glucose sensing is mainly controlled by the diffusion process, the rich specific surface area and carrier density of the heterostructure are critical to the sensitivity of the sensor. The excellent selectivity of the electrode is attributed to the electrostatic repulsion of the heterostructure and interference. In general, the heterostructure provides a higher diffusion area for the reaction [16]. When it is applied to supercapacitors and glucose sensing, it can increase the diffusion rate of reactants and cause faster charge transfer.
4. Conclusions
The three-dimensional core–shell structure NiMn LDH@NiCo2O4/CC electrode was successfully synthesized by the hydrothermal and annealing method. The electrode demonstrates the following characteristics: (1) the active substance is grown directly on the conductive substrate to avoid stripping and ensure the stability of the electrode; (2) synergistic effect of transition metals improves the electrochemical activity; (3) core–shell heterostructure avoids accumulating growth of active substances, shortens the ion/charge transfer path and provides abundant active sites for electrochemical reaction. The NiMn LDH@NiCo2O4/CC electrode possesses excellent electrochemical performance. As a supercapacitor electrode, it shows a high area specific capacitance and outstanding rate capability. In addition, the assembled ASC presents high energy density and superior cycle stability. As a glucose sensor electrode, it also illustrates wide detection range with high sensitivity, low detection limit and remarkable selectivity. The experimental results demonstrate the electrode is a promising material for supercapacitor and glucose sensor.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 51908408, 21872104) and the Natural Science Foundation of Tianjin for Distinguished Young Scholar (No. 20JCJQJC00150).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).