Abstract
2D conjugated polyphthalocyanines can be obtained as two distinctly different types of material with specific molecular structures and different morphological properties. It was believed that the temperature is the key factor affecting the chemical reaction, but we show that even at the optimal temperature (420 °C), the reaction on vapor/solid interface and liquid/solid interface yields different products: while the former is well-polymerized and ordered, the latter is amorphous and cross-linked with the typical conjugation scale of single phthalocyanine (PC) ring. Infrared spectroscopy is most sensitive tool for identifying the molecular structure, providing the information on polymerization degree, structural uniformity and content of terminal groups. We show that, unlike the ordered polyphthalocyanines, the cross-linked product can be reproducibly obtained as continuous conductive material.
Export citation and abstract BibTeX RIS
Introduction
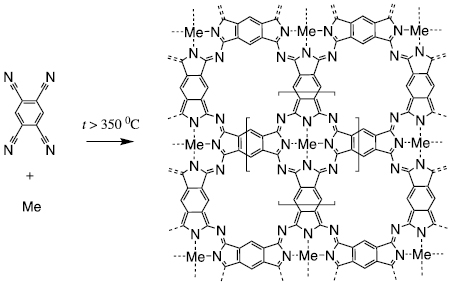
2D conjugated polyphthalocyanines (PPCs) are actively studied as promising materials for next generation batteries [1], advanced fuel cells [2], water splitting catalysts [3], magnetic storage devices [4], spintronics [5] and other applications. Despite the long study history of PPCs [6, 7], there is a controversy in the literature regarding the structure and characterization of these materials. Several groups reported significantly different spectra of the materials produced by different techniques [1, 8–12]. It has been shown recently that the IR, Raman and UV–vis spectra of PPCs strongly depend on the polymerization degree, and products, obtained in many preceding works, should be assigned to either monomeric PPCs or to material of low polymerization degree (oligomers) [13]. A general way to produce PPCs is reaction of pyromellitic tetranitrile with metals or salts at elevated temperature (figure 1), and it was believed that the temperature is the key factor governing the reaction pathway: the formation of polymer needs >350 °C, while at ~200 °C only single ring can be formed (octacyano phthalocyanine, OCP) [11, 12, 14–17]. In the present work, we show that there are other chemical factors strongly affecting the structure and polymerization degree of PPCs. Even at the optimal reaction temperature (~420 °C), two distinctly different products can be obtained depending on the chemical set-up.
Figure 1. General synthetic route to polyphthalocyanines (PPCs): reaction of pyromellitic acid tetranitrile (PMTN) with metal source (Me = Fe, Co, Ni... or H2).
Download figure:
Standard image High-resolution imageIf we use elementary metals (not salts) as a metal source, then the reaction is heterogeneous. We can perform it in two modes: as a vapor/solid and liquid/solid set-up. PMTN has a melting point of ~266 °C, therefore at typical reaction temperatures it should be in a liquid state. Importantly, when heated in atmosphere, pyromellitic acid tetranitrile (PMTN) changes color to blue above 200 °C, which indicates the formation of OCP, a first step of the polymerization [14].
We study the reaction of PMTN with a series of transition metals (Cr, Fe, Co, Ni, Cu) as well as metal-free (H2) and show that the polymerization degree depends on the chemical set-up rather than on the metal.
For a number of microelectronics-related applications, it is desirable to obtain the material in the form of continuous conductive film. This can be difficult, because such conventional processing techniques as spin-coating, sputtering or thermal evaporation cannot be applied to PPCs [9]. We show that morphology and conductivity of PPC films are also influenced by the production method.
Infrared and Raman spectra
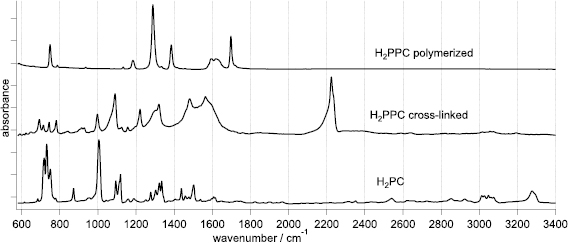
From the IR spectra, we can see significantly different products from the reaction at liquid/solid and at vapor/solid interfaces at the same temperature (at 420 °C), figure 2. While the spectrum after the liquid/solid regime resembles the broadened spectrum of the monomeric PCs, the product from the vapor/solid reaction shows small number of relatively narrow bands at the positions different from those of the monomer. It was proved recently with the help of DFT calculations that the 'true' spectrum of well-ordered polymer is the latter, while the former is poorly-polymerized (cross-linked) product, the electronic conjugation in which has a characteristic scale of single PC ring.
Figure 2. Infrared (IR) spectra of well-polymerized H2PPC from the synthesis at vapor/solid interface (top), cross-linked product from the liquid/solid interface (middle) and the spectrum of the 'monomeric' H2PC (bottom). The cross-linked product has a high signal from the terminal groups (–C ≡ N) at ~2220 cm−1, and the fingerprint region bands are close to those of the monomer, while the ordered polymer has less bands with negligible signal from –C ≡ N groups.
Download figure:
Standard image High-resolution imageContrary to intuition, well-polymerized PPCs lack the characteristic spectral features of monomeric phthalocyanines. Moreover, the spectra of the polymers have significantly less bands than those of the monomeric PC. This is the consequence of the fact that while the PC has 57 atoms in the molecule, the translational unit in the polymer has only 33 atoms (figure 1). In the D4h point group (symmetry of PC molecule and of perfect sheet of 2D polymer), there are total 36 symmetry-allowed bands in the infrared (IR) spectrum of the monomer as compared to 22 in that of the polymer. Not all of these bands have high intensity, and not all of them fall in the fingerprint region, which makes the IR spectrum of PPC quite simple [13].
Similarity between the spectra of cross-linked PPCs and monomeric PCs is an evidence of low polymerization degree. Another important feature is high intensity of terminal groups (–C ≡ N) at ~2220 cm−1 in the spectra of cross-linked material as compared to the ordered one (figure 2). In terms of the structure uniformity, this means a high number of lattice defects in the former. In the ordered PPCs, the 2220 cm−1 band has a negligible intensity and can hardly be detected for most of the experiments. This is a signature of a high polymerization degree.
The bandwidths of cross-linked and ordered polymers reflect well this structure difference: the bands of the ordered material are much narrower (characteristic FWHM is about 15 cm−1).
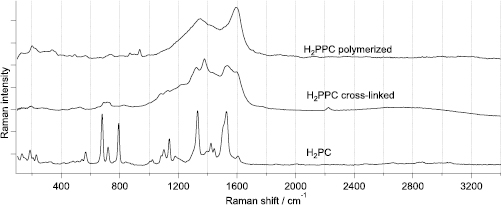
Raman spectra of PPCs are significantly different from those of the monomeric PCs (figure 3). This difference results from the electronic structure: the 2D conjugated π-electronic system of PPCs resembles that of patterned graphene; electronic conjugation is likely to create a resonant Raman effect. The cross-liked product shows intermediate spectral pattern between the polymerized one and the monomer. The resonance however is likely to enhance the cross-section of more conjugated structure fragments, therefore the similarity between the conjugated and cross-liked products is more pronounced in Raman spectra then in the IR. DFT calculations confirm that the well-polymerized PPCs should give several intense close-lying bands in higher fingerprint region [13].
Figure 3. Raman spectra of well-polymerized H2PPC from the synthesis at vapor/solid interface (top), cross-linked product from the liquid/solid interface (middle) and the spectrum of the 'monomeric' H2PC (bottom). The ordered polymer shows broad intense signal from the conjugated system of CC and CN double bonds [13], while the spectrum of the cross-linked product is closer to that of the monomer.
Download figure:
Standard image High-resolution imageFigures 2 and 3 shows IR and Raman spectra of H2PPC from liquid/solid and vapor/solid reaction modes as compared to the spectra of the monomeric H2PC. The other studied MePPCs (Me = Cr, Fe, Co, Ni) show analogous spectral patterns in the two set-ups (SI sections 1 and 2). In the fingerprint region, the bands in MePC spectra belong to the vibrations of organic skeleton of the molecule, therefore show only minor difference upon changing the central ion [18]. The spectra of CuPPC are reported elsewhere [9, 12], they follow the same tendency.
TEM
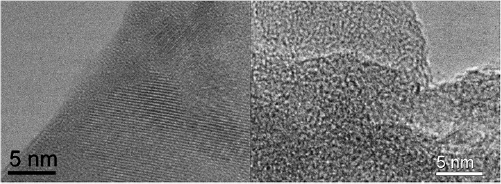
IR spectra show pronounced difference in molecular structure between ordered and cross-linked materials. The corresponding difference at nanoscale is also observed in TEM images (figure 4): the polymerized product shows ordered (crystalline) domains with the typical size of few tens of nm, while the cross-linked product has an amorphous structure.
Figure 4. TEM images of ordered (left) and cross-linked (right) H2PPC materials. The characteristic crystallite size of the ordered polymer is few tens of nm, while the cross-linked product has amorphous structure.
Download figure:
Standard image High-resolution imageFrom the TEM data it is possible to estimate the polymerization degree and characteristic molecular weight. For the ordered polymer, if we consider the macromolecule of ~20 nm size containing 20 × 20 unit cells (1.07 nm from [13]), the molecular weight would be 143 200 a.m.u. (for the metal-free polymer the unit cell is H2 * C20N8H4, 358 a.m.u.). For the disordered polymer, the polymerization degree is about 1–2 unit cells. It is difficult to estimate the cross-linking degree from TEM or spectroscopic data. In the recent work [1] the authors found that the disordered polymer is soluble in organic solvents (DMSO and NMP), from which we can conclude that the real size of the 'cross-linked' macromolecule is relatively small and hardly exceeds few unit cells.
Continuity, conductivity and the growth mechanism
One unexpected finding was that the continuity and conductivity of ordered and cross-linked products was significantly different. The ordered polymer did not always give the conducting film, while the cross-linked material showed well reproducible conductivity. Together with the structural information, this fact provides understanding of the growth mechanism in the two regimes.
In the liquid/solid mode, the kinetic factor plays key role in the growth. The liquid precursor (PMTN) quickly reacts at 420 °C to form the cross-linked product. Therefore, the resulting film retains the morphology of the original PMTN layer as it melts. This makes it continuous and conductive. For metal-free PPC, the liquid/solid growth at 420 °C does not require a catalyst; for MePPCs, the diffusion of metal in the polymer structure is probably a slower step. According to conductivity measurements and TEM studies, free metal clusters are not contained in the cross-linked product after the time of ~1 h. The polymer thickness is controlled by the thickness of the precursor layer on the wafer. The uniform PMTN layer of desirable thickness can be obtained by drop-casting of acetone solution under centrifugation, or by vacuum deposition. Typical thickness of the studied samples ranged from few nm to ~1 µm.
In the vapor/solid regime, the precursor molecules have low concentrations and have enough time to find the thermodynamically favorable point for the growth. This process results in the ordered polymer. The growth proceeds along the crystal facets and/or catalyst particles, which does not always yield the continuous film. An important factor that affects the morphology is the formation of the islands and loss of continuity of the few-nm metal film upon heating. The film thickness in this case is determined by the thickness of the metal film (for MePPCs) or the duration of the synthesis (for H2PPC). The experiments with the copper foil showed that micrometer-scale thickness can be obtained in long experiments [9]. From this we can tell that the metal can easily diffuse through the polymer. The CVD set-up can potentially be modified in order to improve the homogeneity of the films, for example by varying the pressure and/or the precursor supply rate.
The molybdate catalyst for H2PPC is necessary only for the vapor/solid regime. In this case, the polymer film grows selectively only on the molybdate-doped surface [8]. The role of Mo6+ ion is to coordinate the PMTN molecules; this is a common catalyst for industrial synthesis of monomeric PCs (see [19] and references therein). By the steric reasons, the big molybdate ion cannot be incorporated into the PC ring. We however cannot completely exclude some inter-layer and/or defect-site inclusions of molybdate ions in the final structure of the polymer.
Quantum-chemical calculations suggest that MePPCs are low-gap semiconductors with HOCO-LUCO energy difference of 0.02 eV [13]. It was shown that both HOCO and LUCO originate from the π-electrons of the organic skeleton, therefore all MePPCs should have similar electronic properties. In the present work, we performed the electrical measurements for H2PPC and CrPPC. The characteristic values of resistances at room temperature are 3.6 GΩ/square and 1.4 MΩ/square for H2PPC and CrPPC, respectively. For CrPPC, the nearly-linear resistance decrease was observed with increasing temperature. We may conclude that the conductivity has the behaviour expected for low-gap semiconductor. Details on the electric measurements are provided in the supplementary information (stacks.iop.org/JPhysD/52/245303/mmedia) (SI section 3). PPCs show a big promise to a variety of microelectronics applications [1, 2, 4, 5], therefore their electrophysical properties require further study.
Experiment
The vapor/solid synthesis of MePPCs was done according to the previously reported procedure [8, 9]. Briefly, a thin layer (few nm) of metal was deposited on a substrate and placed in a reactor for chemical vapor deposition (CVD). Therein, it was exposed to the PMTN vapor at 420 °C for a few hours. As a substrate, KBr plate or silicon wafer was used. We should emphasize that the vapor/solid reaction is selective even for the metal-free PPC, i.e. the polymer grows only on the catalyst, while the reactor walls remain practically clear.
Deposition of few-nm metal films on the substrate was done with electron-beam evaporation system (Leybold L560), the procedure details are described elsewhere [20]. For H2PPC, instead of metal, we used pre-coating with molybdate catalyst. An aqueous solution (1 g l−1) of ammonium heptamolybdate (99% mass (NH4)6Mo7O24 * 4H2O by Reachem) was drop-casted on a KBr substrate (about 0.05 ml per 5 × 5 mm wafer) and then dried at 150 °C.
For the liquid/solid regime, the same CVD set-up was used. The difference was that the sample with a portion of PMTN was wrapped in aluminum foil instead of exposing to the PMTN vapor; the pressure of hydrogen was maintained at ~1 atm. Molybdate catalyst was found to be not necessary in liquid/solid mode for the case of H2PPC.
In the synthesis, hydrogen plays several roles: remove oxygen, suppress surface oxides and, in case of liquid/solid regime, to maintain the pressure. Both in our experiments and in the literature [1], the polymerization products show the ~1780 cm−1 carbonyl band, unless the oxygen is completely excluded from the reactor.
FTIR spectra were taken with a Bruker Vertex 70 V spectrometer combined with a HYPERION 2000 IR-microscope in the 400–4000 cm−1 range under 1 cm−1 resolution. Raman spectra were measured with a Bruker Senterra micro-Raman system under 532 nm excitation and laser power 2 mW. TEM images were acquired with a JEOL JEM 2100 transmission electron microscope.
Electrical measurements were carried out by SourceMeter Keithley SMU 2450 in RTI optCRYO 105 cryostat cooled with liquid nitrogen. The temperature was controlled by multimeter system Keithley DMM 2701.
Conclusions
Temperature was believed to be the key factor controlling the synthesis of 2D PPCs. In this work we showed however that even at the optimal temperature, the reaction can be performed in two different regimes: heterogeneous (vapor/solid interface) and quasi-homogeneous (liquid/solid interface). These two modes yield two kinds of polymers: ordered and cross-linked, correspondingly. The two products can be easily distinguished by the IR spectra, in which the cross-linked polymer shows spectral features resembling the monomeric PCs with intense signal from terminal groups (–C ≡ N), while the ordered one has less number of bands with narrower bandwidth and lower intensity of terminal groups. Raman spectral patterns of PPCs are also significantly different from those of the monomers due to extension of 2D electronic conjugation.
For the well-ordered PPCs, the growth proceeds along crystal facets and/or catalyst particles, which does not always yield conductive films. On the contrary, the cross-linked PPC films are continuous and show well reproducible conductivity. The material has an electric behaviour of low-gap semiconductor.
Acknowledgments
This work was supported by Russian Science Foundation (Project No. 17-73-10128). TEM studies were carried out on the equipment of the Center for Collective Use in Chernogolovka.