Abstract
Iron oxide nanoparticles (NPs) have been extensively studied in the last few decades for several biomedical applications such as magnetic resonance imaging, magnetic drug delivery and hyperthermia. Hyperthermia is a technique used for cancer treatment which consists in inducing a temperature of about 41–45 °C in cancerous cells through magnetic NPs and an external magnetic field. Chemical precipitation was used to produce iron oxide NPs 9 nm in size coated with oleic acid and trisodium citrate. The influence of both stabilizers on the heating ability and in vitro cytotoxicity of the produced iron oxide NPs was assessed. Physicochemical characterization of the samples confirmed that the used surfactants do not change the particles' average size and that the presence of the surfactants has a strong effect on both the magnetic properties and the heating ability. The heating ability of Fe3O4 NPs shows a proportional increase with the increase of iron concentration, although when coated with trisodium citrate or oleic acid the heating ability decreases. Cytotoxicity assays demonstrated that both pristine and trisodium citrate Fe3O4 samples do not reduce cell viability. However, oleic acid Fe3O4 strongly reduces cell viability, more drastically in the SaOs-2 cell line. The produced iron oxide NPs are suitable for cancer hyperthermia treatment and the use of a surfactant brings great advantages concerning the dispersion of NPs, also allowing better control of the hyperthermia temperature.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Magnetic nanoparticles (mNPs) are a class of NPs that can be manipulated using a magnetic field. These NPs possess unique physical properties and the ability to function at the cellular and molecular levels of biological interactions [1]. Within the last few decades mNPs have been extensively investigated for biomedical applications, such as magnetic resonance imaging contrast agents for diagnosis [1–4] and magnetic hyperthermia agents for cancer treatment [5–9]. Iron oxide NPs are superior to other metal oxide NPs due to their biocompatibility and stability and are the most commonly employed mNPs for biomedical applications [10].
When NPs are in an aqueous medium they may adhere together and form aggregates of increasing size. These aggregates may not only settle out due to gravity but may also change the mNPs' magnetic properties from superparamagnetic to ferromagnetic, due to an increase of particle size [11, 12]. Consequently, the colloidal stability of mNPs is crucial to obtain magnetic colloidal ferrofluids that are stable against aggregation in both a biological medium and a magnetic field [13]. Good stabilization can be achieved by controlling the strength of one or both repulsive forces (electrostatic and steric repulsion). When dealing with superparamagnetic NPs in an aqueous medium, magnetic interaction must also be added [14].
The surface of iron oxide NPs is covered by iron atoms that act as Lewis acids, which coordinate with molecules that donate lone-pair electrons. When iron oxide NPs are placed in an aqueous medium, water dissociates and the iron oxide surface becomes hydroxyl functionalized. These hydroxyl groups are amphoteric and therefore may react with either acids or bases. The surface of the iron oxide NPs may be positive or negative, depending upon the pH of the solution. The isoelectric point for magnetite is around pH 6.8. Around this point of zero charge the surface charge density is too small and the particles are no longer stable in water and flocculate. Accordingly, to obtain stable iron oxide NPs it is necessary to ultilize both electrostatic and steric stabilization [13, 15]. In this regard, surfactants and polymers can be chemically anchored or physically adsorbed on mNPs to form a single or double layer. This coating creates repulsive (mainly as steric repulsion) forces to balance the magnetic and the van der Waals attractive forces. As a consequence, the NPs are stabilized in suspension by steric repulsion [16, 17].
Therefore, in this study we evaluated the influence of two stabilizers, oleic acid and trisodium citrate, on the heating ability of iron oxide NPs when subjected to an ac magnetic field. Oleic acid and trisodium citrate were selected as they are highly efficient stabilizers for iron oxide NPs [18]. Furthermore, in vitro cytotoxicity was also evaluated.
2. Materials and methods
2.1. Iron oxide NP synthesis
All the chemical reagents used in this research were of analytical grade and were used without further purification. Iron oxide NPs were synthetized using the method described elsewhere [18] based on chemical co-precipitation. An amount of trisodium citrate and oleic acid was added to a known volume of the NP colloid after synthesis and the mixture was allowed to react for 1 h under stirring. Finally the samples where sonicated for 5 min before use.
The iron concentration of the samples varied from 5 mM to 376 mM, measured using the 1,10-phenanthroline colorimetric method [19]. The surfactant influence was analysed by changing the concentration within the range of 0 to 30 mM and 0 to 64 mM of trisodium citrate and oleic acid, respectively, in four different iron concentrations ([Fe] = 32.6, 60.2, 125.3, 376 mM).
2.2. Characterization
The crystalline phases of the samples were verified using powder x-ray diffraction (XRD). An X'Pert PRO PANAlytical x-ray diffractometer was used to obtain XRD patterns of the iron oxide NPs which had been freeze dried. The 2θ values were taken from 15° to 80° using Cu–Kα radiation (k = 1.54060 Å) with a step size of 0.033.
To investigate changes in the chemical bonds of the samples infrared spectrometry was used. Fourier transform infrared (FTIR) spectra of the samples were obtained using a Nicolet 6700—Thermo Electron Corporation Attenuated Total Reflectance-Fourier Transform Infrared spectrometer. Measurements were performed in freeze dried samples in the range of 480 to 4500 cm−1.
Transmission electron microscopy (TEM) images were obtained using a Hitachi H-8100 II with thermionic emission LaB6. A diluted suspension of NPs was placed in a Kevlar 25 mesh grid for analysis.
Hydrodynamic size was measured using a SZ-100 nanopartica series (Horiba, Lda) with a laser of 532 nm. Measurements were carried out for diluted NP suspensions in triplicate using a disposable cell.
The dc magnetic properties were assessed using a 7 T SQUID magnetometer (S700X; Cryogenic Ltd). The zero-field cooled (ZFC) and FC measurements were performed by cooling the sample to 5 K at zero field or in the presence of an external dc field of 100 Oe, respectively. All the magnetic measurements were carried out in an increasing temperature range of 5–320 K. Isothermal magnetization curves were obtained for dc fields up to 5 T for temperatures of 10 K and 320 K.
The rheological characterization of the agar 1% wt. was performed using a stress controlled rheometer Bohlin Gemini HR nano with parallel plate geometry (20 mm diameter). The evolution of G', G'' and complex viscosity were measured in a temperature sweep oscillatory test (20 °C–70 °C) at a non-destructive frequency of 1 Hz and within the linear viscoelastic regime previously determined from strain sweep tests (not shown). The evolution of the viscosity with temperature (25 °C–70 °C) at a constant shear rate (0.02 s−1) was also assessed in a continuous oscillation mode.
2.3. Magnetic hyperthermia measurements
Hyperthermia measurements were obtained using a DM100 series from Nb Nanoscale Biomagnetics apparatus. This apparatus allows measurements at different magnetic field intensities up to 24 kA m−1 with a fixed frequency of 418.5 kHz. The heating ability of a 1 ml sample of freshly prepared NP solution was measured under different settings. To evaluate the effect of concentration on the thermal behaviour of pristine Fe3O4 colloids, measurements were performed over 40 min maintaining a constant magnetic field intensity and frequency. Next, to evaluate the field intensity effect, different concentrations were tested for a period of 10 min while maintaining the frequency as constant.
To study the influence of both oleic acid and trisodium citrate on heating ability, NP samples with different NP concentrations were subjected to magnetic field intensities from 8 to 24 kA m−1, varying the surfactant concentration.
Finally, samples for in vitro heat generation were prepared. NP colloids were dispersed in a hot agar solution (1 wt. percentage of agar) in a glass vial. Samples were sonicated for 1 min to disperse the NPs and then solidified at room temperature. The iron content of all the samples was kept constant as 94 mM of iron. The agar phantoms were subjected to an ac magnetic field of 24 kA m−1, with a frequency of 418.5 kHz for 10 min.
The overall study is summarized in table 1.
Table 1. Tested conditions to evaluate the influence of the stabilizers (oleic acid (OA) and trisodium citrate (TC)) on the heating ability of the iron oxide colloids.
| Substrate | Magnetic field strength (kA m−1) | Time of field application (minutes) | Iron concentration (mM) | Stabilizer concentration (mM) | |
|---|---|---|---|---|---|
| Pristine Fe3O4 | Water | 24 | 40 | [0.4–376] | — |
| 10 | [5.4–107] | ||||
| 8, 12, 18, 24 | 10 | [32.6–376] | |||
| Agar | 24 | 10 | 94 | ||
| Fe3O4 OA | Water | 24 | 10 | [5.4–107] | 64 |
| 8, 12, 18, 24 | 10 | [32.6–376] | [0–64] | ||
| Agar | 24 | 10 | 94 | 64 | |
| Fe3O4 TC | Water | 24 | 10 | [5.4–107] | 10 |
| 8, 12, 18, 24 | 10 | [32.6–376] | [0–30] | ||
| Agar | 24 | 10 | 94 | 10 |
2.4. Cytotoxicity assay
Cell viability studies were performed using a resazurin assay on Vero (a fibroblast-like kidney cell) and SaOs-2 (primary osteogenic sarcoma) cell lines. The cells were seeded at a density of 5 × 104 cells ml−1 in 96-well plates. The SaOs cells were grown in McCoy 5A medium, supplemented with 10% foetal bovine serum and 1% penicillin–streptomycin (10 000 U mL−1), while the Vero cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, 1% penicillin–streptomycin (10 000 U mL−1), sodium pyruvate (100 mM) and GlutaMAX™ supplement followed by incubation at 37 °C in 5% CO2 overnight. The next day, the cells were treated with a fresh medium containing a known concentration of NPs ranging from 12.5 mM to 0.78 mM Fe in each well in triplicate and the plates were incubated for 24 h. After this time, the medium was removed and rezasurin was added to each well. After a 2 h incubation time the absorbance was measured at 570 and 600 nm. Control cells were treated similarly and were incubated with the respective medium at the same dilution as the one used for incubation with NPs. Cell viability was expressed as a percentage of the control, given by [% cell viability = NP treated cells/control cells × 100].
3. Results and discussion
3.1. Physicochemical properties
Figure 1(A) shows the XRD patterns obtained for pristine iron oxide NPs. The six characteristic peaks occurred at 2θ of 30.1, 35.5, 43.2, 53.5, 57.0 and 62.8, which are marked by their corresponding indices (220), (311), (400), (422), (511) and (440), respectively. Comparison of the XRD pattern of the NPs with the standard diffraction spectrum for magnetite powders (JCPDS 00-019-0629) for the synthesized product we clearly identify the diffraction peaks of crystalline cubic magnetite structure. The average grain size was calculated to be 9.75 nm using Scherrer's equation: τ = Κλ/βcosθ where τ is equivalent to the grain average core diameter, K is the grain shape factor (K = 0.94), λ is the incident x-ray wavelength, β denotes the full width at half-maximum (in radians) of the highest intensity and θ is the corresponding diffraction angle (2θ = 35.6141). The stabilized iron oxide NPs retained the crystal structure of pristine Fe3O4, as reported elsewhere [18], showing that the stabilizer does not change the Fe3O4 NPs' crystalline structure.
Figure 1. (A) X-ray patterns of pristine iron oxide NPs produced using the chemical precipitation technique. (B) FTIR spectra of pristine Fe3O4 NPs (Fe3O4), trisodium citrate coated Fe3O4 NPs (TC 30 mM), and oleic acid coated Fe3O4 NPs (OA 64 mM).
Download figure:
Standard image High-resolution imageFTIR measurements were performed for pristine and coated Fe3O4 NPs (figure 1(B)). In the pristine NP spectrum typical absorbance bands for Fe3O4 are present: the bands at 560 cm−1, 1630 cm−1, 3000 cm−1 and 3500 cm−1 are attributed to the Fe–O stretching vibration mode, O–H stretching vibration mode and O–H stretching vibration mode due to water vapour, respectively [20].
In addition to the Fe3O4 typical absorbance bands, the FTIR spectra of trisodium citrate and oleic acid coated Fe3O4 NPs present the typical bands for each stabilizer. For oleic acid coated NPs the bands at 2910 and 2840 cm−1 are attributed to the symmetric and asymmetric CH2 stretch in the oleic acid molecule, respectively. The band at 1415 cm−1 is due to the asymmetric –COO- stretch vibration mode; a sharp band at 1704 cm−1 corresponds to the stretching vibration of C=O in oleic acid. In the trisodium citrate Fe3O4 NPs the absorptions bands at 1369 and 1568 cm−1 are characteristic of the COO–Fe bond which may be due to the reaction of the hydroxide radical groups on the surface of Fe3O4 with the carboxylate anion of trisodium citrate [21]. These characteristic bands prove that the trisodium citrate molecule is linked to the NP surface, thus providing stability in aqueous solutions.
A TEM image of pristine iron oxide NPs shows an average diameter of 8.5 nm and an important aggregation between the NPs (figures 2(A), (B)). The obtained size correlates with the average particle size obtained from Scherrer's equation. TEM images of oleic acid 96 mM and trisodium citrate 30 mM, figures 1(C) and (D), respectively, were taken to evaluate the influence of higher concentrations of surfactants on the iron oxide core. The TEM images show that surfactants do not contribute to a change in the size of the iron oxide NPs' core.
Figure 2. TEM images of pristine Fe3O4 (A) and the respective size distribution (B), oleic acid 96 mM (C) and trisodium citrate 30 mM (D). The insets in (C) and (D) correspond to the respective size distributions.
Download figure:
Standard image High-resolution imageIn order to investigate the influence of surfactants on the NPs' hydrodynamic size we have chosen the most promising concentrations of both surfactants and measured their hydrodynamic sizes and zeta potentials. The results are shown in table 2. Although there is a slight difference between the samples it is possible to see that the mean hydrodynamic size of the NPs does not significantly change with the addition of surfactants. However, the hydrodynamic size of the NPs increases with a higher concentration of oleic acid. This increase is due to the NPs' bilayer coating of oleic acid as previously observed [18].
Table 2. Properties of pristine iron oxide NPs and NPs coated with a low and a higher concentration of both trisodium citrate (TC) and oleic acid (OA): average TEM diameter, average hydrodynamic diameter and magnetic properties—magnetic saturation (MS) and blocking temperature (TB).
| Sample | Average diameter (TEM) (nm) | DH (nm) | Zeta potential (mV) at pH 9 | MS (emu g−1) | TB (K) |
|---|---|---|---|---|---|
| Pristine Fe3O4 | 8.5 ± 2.0 | 176.0 | −105.2 | 58 | 172 |
| Fe3O4 TC 10 mM | – | 127.5 | −95.4 | 60 | 157 |
| Fe3O4 TC 30 mM | 9.1 ± 2.2 | 136.6 | — | 60 | 147 |
| Fe3O4 OA 16 mM | 9.0 ± 1.9a | 145.9 | −66.8 | 34 | 162 |
| Fe3O4 OA 64 mM | 8.5 ± 2.0a | 220.0 | −126.8 | 45 | 147 |
3.2. Magnetic characterization
The dc magnetic properties of pristine and coated iron oxide NPs were evaluated. In all cases, magnetic saturation is represented as emu per gram of the whole particle (including the magnetic and non-magnetic material). Pristine iron oxide NPs have a saturation magnetization (MS) of around 59 emu g−1 at 320 K. While trisodium citrate does not change the magnetic saturation of the NPs, oleic acid reduces the MS of the NPs (around 45 emu g−1 for a surfactant concentration of 64 mM).
The blocking temperature (TB) of the samples was determined as the maximum value of the ZFC curve. The temperature dependence of the magnetization of pristine Fe3O4 NPs exhibits a TB around 172 K, which indicates that at room temperature the produced NPs have a superparamagnetic behaviour. Although the obtained blocking temperature (TB) for trisodium citrate and oleic acid coated Fe3O4 NPs shifts slightly, it still remains in the same order of magnitude. Therefore, stabilized Fe3O4 NPs with either oleic acid or trisodium citrate remain superparamagnetic at room temperature.
Table 2 summarizes the magnetic characterization results.
Figure 3 shows a magnification of the hysteresis loops of pristine Fe3O4 measured at 10 K and 320 K (figure 3(A)), and of trisodium citrate Fe3O4 NPs (figure 3(B)) and oleic acid Fe3O4 NPs (figure 3(C)) at 320 K. In all cases the absence of coercivity and remanence at 320 K is evident. The magnetic domains are not separated by domain walls (a multi-domain state). Instead, each particle represents a single magnetic domain [22]. For magnetite the critical size is 30 nm at room temperature [23]. This observation confirms the superparamagnetic behaviour of our samples. The loop areas for all samples are narrow, indicating very low heat generation due to hysteresis, which also correlates with the literature [22, 24].
Figure 3. Magnification of hysteresis loops of (A) pristine iron oxide NPs measured at 10 K and 320 K, and (B) trisodium citrate (TC) and (C) oleic acid (OA) stabilized Fe3O4 NPs measured at 320 K.
Download figure:
Standard image High-resolution image3.3. Hyperthermia measurements
In the first step the hyperthermia capability of several concentrations of the uncoated NP colloids without surfactant were tested for a frequency of 418.5 kHz, an ac magnetic field intensity of 24 kA m−1, 1 ml of the colloid and 40 min of field application. All the heating curves were adjusted to a mathematical model (equation (1)) of the ZAR v1.0 software from Nb nanoscale Biomagnetics,
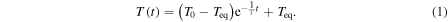
In the equation T0 is the initial temperature of the colloid, Teq is the equilibrium temperature of the colloid, T is the maximum temperature reached by the colloid and τ is the characteristic time of heating.
Figure 4 represents the temperature variation of the uncoated iron oxide colloids as a function of iron concentration obtained using the above stated experimental conditions. The red line is an exponential adjustment to the obtained data. This exponential adjustment demonstrates that the heating ability of the NPs tends to decrease for high NP concentration according to the empirical equation:
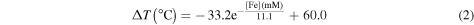
with a correlation factor (R2) of 0.995.
Figure 4. Temperature variation generated by pristine Fe3O4 during 40 min of ac magnetic field application with an intensity of 24 kA m−1 and 418.5 kHz as a function of iron concentration.
Download figure:
Standard image High-resolution imageThe exponential adjustment to the experimental data suggests an increase of heat as the NP concentration increases with a tendency to saturate at a very high iron concentration. This is correlated to agglomerates and as the superparamagnetic NPs show weak dipole–dipole interaction the exchange interactions dominate but the agglomerates behave like a particle, with an effective volume larger than a single core. As such, the agglomerate behaves like a ferromagnetic particle.
Figure 5 represents the temperature variation as a function of iron concentration for the optimal surfactant concentrations. These experiments were performed for 10 min, with an ac magnetic field intensity of 24 kA m−1 and a frequency of 418.5 kHz.
Figure 5. Generated temperature of pristine Fe3O4 (black dots), trisodium citrate (TC) 10 mM (red dots) and oleic acid (OA) 64 mM (green dots) stabilized NPs during 10 min of ac magnetic field application with an intensity of 24 kA m−1 and 418.5 kHz of frequency as a function of iron concentration.
Download figure:
Standard image High-resolution imageSimilarly to what can be observed in figure 4, we can verify that the pristine Fe3O4 heating ability tends to stabilize for high iron concentrations as expressed by the empirical equation ![${\rm{\Delta }}T=-46.3{{\rm{e}}}^{-\frac{\left[{\rm{F}}{\rm{e}}\right]}{66.2}}+47.1.$](https://content.cld.iop.org/journals/0957-4484/26/42/425704/revision1/nano519917ieqn1.gif) However, when the surfactant is present this effect is no longer observed and the best fitting equation is
However, when the surfactant is present this effect is no longer observed and the best fitting equation is ![${\rm{\Delta }}T=0.27\left[{\rm{F}}{\rm{e}}\right]+0.53$](https://content.cld.iop.org/journals/0957-4484/26/42/425704/revision1/nano519917ieqn2.gif) for oleic acid and
for oleic acid and ![${\rm{\Delta }}T=0.43\left[{\rm{F}}{\rm{e}}\right]+1.93\;$](https://content.cld.iop.org/journals/0957-4484/26/42/425704/revision1/nano519917ieqn3.gif) for trisodium citrate. The surfactant reduces the aggregation and so the heating ability is still efficient even for high concentration of iron NPs. However, if the normal body temperature is considered, a temperature variation of five degrees is enough to achieve the hyperthermia range of temperature (around 42 °C). Moreover, within the tested conditions, an iron concentration above 10 mM for pristine and trisodium citrate Fe3O4 and 20 mM for oleic acid Fe3O4 is enough to generate the required temperature.
for trisodium citrate. The surfactant reduces the aggregation and so the heating ability is still efficient even for high concentration of iron NPs. However, if the normal body temperature is considered, a temperature variation of five degrees is enough to achieve the hyperthermia range of temperature (around 42 °C). Moreover, within the tested conditions, an iron concentration above 10 mM for pristine and trisodium citrate Fe3O4 and 20 mM for oleic acid Fe3O4 is enough to generate the required temperature.
The influence of the surfactants on the heating ability of the NPs was tested with four iron concentrations, 32.6, 60.2, 125.3 and 376 mM, and also four different applied ac magnetic field intensities, 8, 12, 18 and 24 kA m−1. Both the frequency and time of the experiment were fixed to 418.5 kHz and 10 min, respectively.
Specific absorption rate (SAR) is used to characterize the heating efficiency of a magnetic material through energy absorption during its exposure to an alternating magnetic field. The value is defined as the quantity of power absorbed by the sample per mass unit (W/g) and was calculated using the following equation:
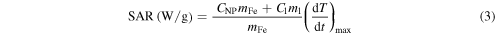
where (dT/dt)max is the maximum gradient of the temperature curve of the colloid submitted to an hyperthermia test, CNP is the specific heat of the NPs, Cl is the specific heat of the liquid, ml is the fluid mass and mFe is the iron mass in the colloid.
An accurate estimation of the SAR values of magnetic colloids must be taken under adiabatic conditions. Therefore, to calculate the SAR values we used the maximum of the derivative dT/dt. Since maximum increase always occurs in the firstfew seconds of the experiments the adiabatic conditions are secured [24].
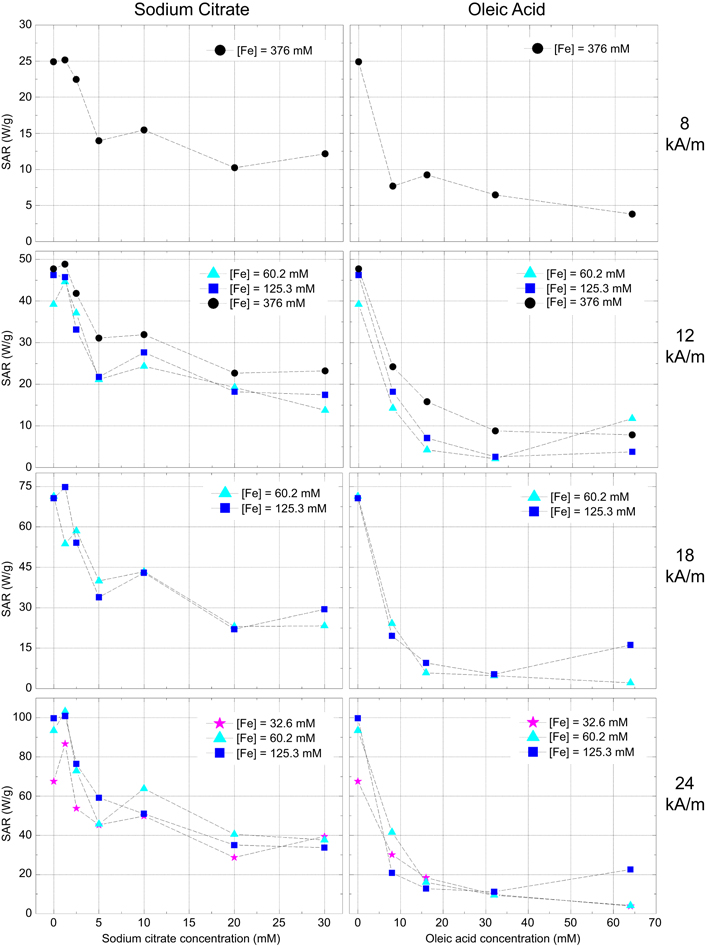
SAR values as a function of the surfactant concentration are shown in figure 6 for the four different magnetic field intensities and different iron concentrations.
Figure 6. SAR values as a function of surfactant concentration for different iron concentrations at 8, 12, 18 and 24 kA m−1 of the ac magnetic field strength, with a fixed frequency of 418.5 kHz.
Download figure:
Standard image High-resolution imageThe results of figure 6 clearly show that the addition of surfactants, even in concentrations as low as 5 mM for trisodium citrate or 10 mM for oleic acid, strongly reduces the SAR values to values below 60 W g−1 and 20 W g−1, respectively. Moreover, this behaviour is observed independently of the iron concentration or magnetic field applied. However, a small increase in the SAR value is obtained for concentrations bellow 2 mM of trisodium citrate. This increase depends on the iron concentration and on the magnetic field applied.
In 2002, Rosensweig [25] developed relationships based on rotational relaxation of single domain magnetic particles dispersed in a liquid matrix. The magnetic material when submitted to an ac magnetic field exhibits both Brownian and Néel relaxations. Nevertheless, Néel relaxation must not be allowed to dominate. The expressions of Brownian (equation (4)) and Néel (equation (5)) relaxations times are
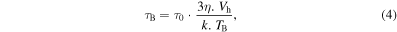
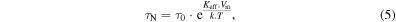
where η is the viscosity of the carrier liquid, Vh is the effective hydrodynamic volume and Vm is the particle volume, k is the Boltzmann constant, TB is temperature, Keff is the magnetic anisotropy and τ0 is the characteristic flipping time [22]. These relaxation times are related to the vanishing of the remnant magnetization once the applied ac magnetic field is removed.
When the NPs are in suspension both relaxation paths are present. Furthermore, Brownian relaxation occurs due to the ability of the particles to rotate freely. This relaxation mechanism is mainly due to the NPs' hydrodynamic volume and the viscosity of the carrier fluid as in equation (3). However, when the NPs are injected into the body or directly into the tissue the NPs are up taken by tumour cells and stay fixed. In this situation no Brownian relaxation may occur when an ac magnetic field is applied [22].
Considering this, we have performed magnetic hyperthermia tests in agar phantoms containing a known concentration of NPs. Figure 7 shows that the SAR values are lower for lower iron concentrations of pristine iron oxide NPs, which is related to the aggregation of pristine iron oxide NPs, affecting their heating generation ability as previously discussed. For pristine and trisodium citrate stabilized Fe3O4 NPs, the SAR values decrease about 50% compared to the ones obtained for water. The agar phantoms cause the entrapment of the NPs, eliminating their Brownian movements. Therefore, heat generation can only be attributed to Néel relaxation. However, for oleic acid stabilized NPs the SAR value is almost the same as the one of water dispersed NPs. This is related to the bilayer formed around the iron oxide cores where oleic acid has a similar effect to agar phantoms, entrapping the NPs and eliminating their Brownian movements, which can also explain the low MS observed in these samples.
Figure 7. Comparison of the SAR values of pristine and coated NPs in agar phantoms and water. The results are expressed as the average ± standard deviation for three independent experiments. *p < 0.05 compared with the respective agar sample.
Download figure:
Standard image High-resolution imageThe Brownian and Néel relaxation times were both estimated in order to confirm the above assumptions. For that, we used the TEM diameters to calculate the particle magnetic volume (Vm), the magnetic anisotropy constant (Keff) of approximately 3 kJ m−3 [26] and a characteristic flipping time (τ0) of 10−9 [27]. Water viscosity (η) was considered to be 1 × 10−3 Pa.s and agar 1% wt. viscosity was determined to be 4.3 × 103 Pa.s. The corresponding results are presented in table 3.
Table 3. Estimated Brownian (τB) or viscous and Néel (τN) or magnetic relaxation times for pristine and trisodium citrate (TC) 10 mM and oleic acid (OA) 64 mM iron oxide NPs in both water and agar 1% wt.
| Sample | Substrate | τN (s) | τB (s) |
|---|---|---|---|
| Pristine Fe3O4 | Agar | 1.43 × 10−8 | 1.01 × 10−5 |
| Water | 2.36 × 10−11 | ||
| Fe3O4 TC 10 mM | Agar | 7.57 × 10−8 | 6.26 × 10−6 |
| Water | 1.46 × 10−11 | ||
| Fe3O4 OA 64 mM | Agar | 6.78 × 10−8 | 2.55 × 10−5 |
| Water | 5.96 × 10−11 |
Goya et al [26] have proposed a versatile diagram to identify the dominant heating mechanism of magnetic single domains in magnetic fluid hyperthermia. This diagram considers the magnetic mechanism of relaxation (Néel relaxation) and the viscous mechanism of relaxation (Brownian relaxation). The probabilities of viscous and magnetic relaxation are proportional to 1/τB and 1/τN, respectively. In this diagram they have identified three regions: (1) τB < 0.1τN where the viscous mechanism is dominant (viscous region), (2) τN < 0.1τB where the magnetic mechanism is dominant and (3) τN ∼ τB where both mechanisms contribute to heat generation. Analysing the results in table 3 for the relaxation times of both Brownian and Neel relaxation it is clearly visible that when the NPs are dispersed in water the Brownian mechanism is dominant. In this case, the NPs are able to rotate freely and so the viscous mechanism is dominant over the magnetic mechanism. However, when the NPs are dispersed in the agar gel, they are entrapped in the gel and so their rotation is limited. In this case the Néel mechanism is clearly dominant.
For pristine and trisodium citrate iron oxide NPs the reduction in the SAR value is probably due to this change in the dominant relaxation mechanism. However, for oleic acid this difference is not observed. In this case, although the viscosity of the fluid is not clearly affected, the coating acts as an entrapment for the iron oxide cores, eliminating their mobility and consequently decreasing the influence of the Brownian relaxation mechanism for the effective relaxation time of the NPs.
3.4. Cytotoxicity assay
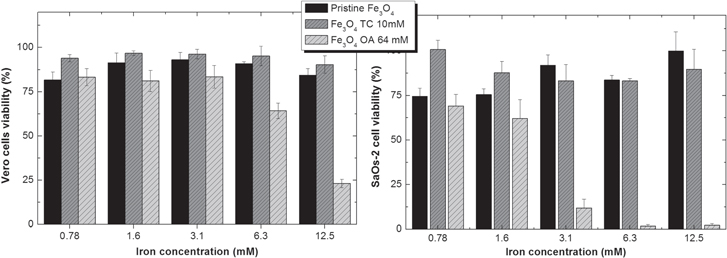
The cytotoxicity results on the cell lines Vero and SaOs-2 are shown in figure 8. The chosen cell lines were intended to evaluate the cytotoxic effect of the produced NPs in a 'normal' (Vero) and a tumour (SaOs-2) cell line. The results are expressed in % of cell viability calculated by [% cell viability = NP treated cells/control cells × 100]. The results show that both the Vero and SaOs cell lines do not show any toxicity to pristine and trisodium citrate Fe3O4 NPs in the range of 0.78–12.5 mM Fe. However, for oleic acid Fe3O4 the cytotoxicity profile is different. For Vero cells and iron concentrations above 6.3 mM cell viability is reduced, thus causing cytotoxic effects. For SaOs-2 cells, cell viability is reduced for the tested range of iron concentration, although the cytotoxic effect is mild for iron concentrations of 0.78 and 1.6 mM.
Figure 8. Cytotoxicity profiles of pristine Fe3O4, and trisodium citrate (TC) and oleic acid (OA) coated Fe3O4 on Vero and SaOs-2 cells.
Download figure:
Standard image High-resolution image4. Conclusions
Iron oxide NPs are one of the most studied materials for several biomedical applications due to their unique properties. One of the biggest concerns for these nanostructures is their stability in aqueous solutions. In this work, iron oxide NPs prepared by a co-precipitation method were stabilized using trisodium citrate and oleic acid as surfactants. We have clearly identified that both surfactants do not significantly change the core size of the NPs, while the hydrodynamic size of the NPs increases with higher concentrations of oleic acid. Moreover, both the magnetic properties and the heating ability of the colloids are dependent on the surfactant used. Based on the obtained results, we suggest that oleic acid acts as a retention agent of the iron oxide cores preventing their Brownian movements. Thus, oleic acid stabilized NPs results may be more closely related to the in vivo situation where NPs are entrapped in cells.
The temperature profiles (i.e. temperature evolution with time) of the NPs in the presence of an ac magnetic field revealed that the two studied colloids are suitable for cancer hyperthermia and that the SAR values can be controlled by the concentration of surfactants. These results may contribute to improving the stability of iron oxide colloids and to correctly assess the influence that both trisodium citrate and oleic acid have on the NPs' magnetic and thermal properties, thus promoting their biomedical application.
Acknowledgments
This work is funded by FEDER funds through the COMPETE 2020 Programme and National Funds through FCT—Portuguese Foundation for Science and Technology under the project UID/CTM/50025/2013. PIPS and JTC acknowledge FCT for PhD grants, SFRH/BD/81711/2011 and SFRH/BD/84628/2012, respectively. The authors wish to thank Dr César Laia for the dynamic light scattering measurements.