-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew F. Shorr, Neringa Zadeikis, William L. Jackson, Anthony S. Ramage, Shu-Chen Wu, Alan M. Tennenberg, Marin H. Kollef, Levofloxacin for Treatment of Ventilator-Associated Pneumonia: A Subgroup Analysis from a Randomized Trial, Clinical Infectious Diseases, Volume 40, Issue Supplement_2, February 2005, Pages S123–S129, https://doi.org/10.1086/426192
Close - Share Icon Share
Abstract
Ventilator-associated pneumonia (VAP) remains a significant challenge in critical care. We conducted a secondary analysis of a multicenter, prospective, randomized trial comparing levofloxacin (750 mg iv q24h) with imipenem-cilastatin (500–1000 mg iv q6–8h) for treatment of nosocomial pneumonia and focused on the subgroup of patients with VAP. The study cohort included 222 patients, with half (111) of the patients assigned to each treatment group. The patients in both groups were similar with respect to age, severity of illness, and duration of mechanical ventilation before the onset of VAP. Among the intention-to-treat population, clinical success was achieved in 58.6% of patients receiving levofloxacin, compared with 63.1% of patients receiving imipenem-cilastatin (P = .49; 95% confidence interval for the difference, -8.77% to 17.79%). Microbiological success and 28-day mortality rates were also comparable. Multivariate analysis demonstrated that assignment to antibiotic treatment (i.e., levofloxacin vs. imipenem-cilastatin) was not predictive of outcomes, thus suggesting that the treatment regimens were equivalent. Both levofloxacin and imipenem-cilastatin regimens were well tolerated and had similar adverse event profiles.
Ventilator-associated pneumonia (VAP) is a frequent nosocomial infection. Among critically ill patients, it is the second most common nosocomial infection but is the leading cause of nosocomial morbidity [1, 2]. Development of VAP increases both the duration of mechanical ventilation (MV) and the length of stay in the intensive care unit (ICU) [3, 4]. Some researchers estimate that the attributable cost per case of VAP is ∼$12,000 [5, 6]. The effect of VAP on mortality is less clear, but the mortality rate attributable to VAP may approach 30% [7].
Many empirical regimens for treating VAP include either an antipseudomonal cephalosporin, carbapenem, or an extended-spectrum penicillin combined with either an aminoglycoside or a quinolone [4]. Quinolones are now commonly used to treat VAP because of their excellent penetration into epithelial lining fluid and because of their favorable pharmacodynamic profile [8, 9]. Quinolones also lack the potential for significant nephrotoxicity, unlike aminoglycosides. Nonetheless, few formal trials of either quinolones or aminoglycosides have been conducted in the context of VAP [10, 11]. In addition, the majority of randomized studies exploring the efficacy of these agents have involved mixed populations of patients with nosocomial pneumonia (NP) [10, 11]. As such, it is often difficult to discern in the reports of these trials the efficacy of any of these antibiotics in treating VAP per se. Increasing rates of resistance to quinolones among Pseudomonas species and other gram-negative pathogens are also raising concern [12]. These changes in drug-resistance patterns further underscore the need for outcome data associated with VAP so that clinicians can have information from clinical trials that directly pertain to the patients they are treating.
A recently completed randomized trial compared levofloxacin with imipenem-cilastatin for treating NP [13]. To provide more-detailed information about the efficacy of these agents and the outcomes from this trial involving patients with VAP, we conducted a subgroup analysis specifically exploring clinical success rates and mortality in the cohort of patients with VAP. Before this subgroup analysis, we formally hypothesized that levofloxacin would not be inferior to imipenem-cilastatin for treating VAP.
Methods
Study overview. The study was a multicenter, open-label, comparator-controlled randomized trial. The methods of this trial have been described in detail elsewhere [13]. In brief, patients who met eligibility criteria (see below) were randomized in a 1 : 1 fashion to receive either levofloxacin (750 mg iv q24h) or imipenem-cilastatin (500–1000 mg iv q6–8h). Dosing was adjusted for renal impairment. Combination therapy was administered for cases of suspected infection with Pseudomonas aeruginosa. For patients receiving levofloxacin, the additional agent was ceftazidime (2 g iv q8h) or another noncarbapenem β-lactam; for patients receiving imipenem-cilastatin, the additional agent was amikacin (7.5 mg/kg iv q12h) or an alternative aminoglycoside. In suspected or documented cases of infection with methicillin-resistant Staphylococcus aureus, investigators used vancomycin, irrespective of study treatment.
Patients and populations. Patients >18 years old with signs and symptoms of NP were eligible for the original trial. Patients had to have been hospitalized for >72 h, and radiographic evidence of a new infiltrate was required. The presence of either abnormal body temperature (⩾38°C or ⩽35°C) or an abnormal peripheral leukocyte count (⩾12,000 cells/mm3, >10% immature forms, or ⩽3500 cells mm3) was further required for enrollment in the study. Patients with neutropenia (⩽500 neutrophils/mm3) were excluded. We defined“VAP,” the focus for the present report, as the development of pneumonia in a patient who had been receiving MV for at least 48 h before the development of a new infiltrate, accompanied by the evolution of other signs and symptoms of pneumonia. Patients who acquired VAP after ⩾6 days of MV were categorized as having late-onset VAP. Among patients with VAP, outcomes were assessed for 3 populations: intention-to-treat (ITT; i.e., all enrolled patients), clinically evaluable (i.e., all patients who had VAP and no protocol violations during study), and microbiologically evaluable populations (i.e., patients with microbiologically proven infection who had no protocol violations).
Outcomes and end points. The primary end point for the analysis was clinical success in the ITT population. Clinical success was represented by either cure or improvement, with “cure” defined as complete resolution of signs and symptoms of VAP. Partial resolution, such that no further antimicrobial therapy was needed, indicated “improvement.” Clinical success was also determined in the clinically evaluable and microbiologically evaluable cohorts. To be conservative, patients lost to follow-up were categorized as having experienced treatment failure in the ITT population. Death occurring during the 28 days after enrollment served as a secondary end point. The emergence of resistance among and rates of superinfection with Pseudomonas species also represented secondary end points. The emergence of resistance was defined as isolation, at the end of therapy, of the same pathogen recovered initially but with newly acquired resistance to the study agent the patient received. Superinfection was defined as recovery of a new organism, from specimens from any site, while the patient was receiving therapy, up to and including the posttherapy culture. To minimize the potential of misconstruing colonization as superinfection, we required recovery of the new organism to be associated with the emergence or worsening of clinical signs and symptoms and/or with laboratory evidence of active infection and antimicrobial therapy to be necessary. For safety analysis, we recorded the incidence of serious adverse events (SAEs) as noted by investigators, as well as rates of study agent discontinuation.
Statistics. Continuous data are reported as the mean α SD, whereas categorical data are shown as the percentage of the study population. Continuous data were compared by use of the Student's t test, whereas categorical data were analyzed by use of a χ2 test. P < .05 was assumed to denote statistical significance; 95% CIs are reported where appropriate.
Since this was a subgroup assessment of a larger trial, we conducted a multivariate analysis to control for potential differences between the 2 treatment populations in terms of identifying predictors of clinical success. In an initial model, variables that were statistically significant (P < .2) in univariate analysis were placed into a stepwise, forward, multivariable logistic regression, with adjustments made for colinearity. Treatment assignment (levofloxacin vs. imipenem-cilastatin) was retained in this model. In a second model, all recorded variables, even if they were not statistically significant (P < .2), were placed into a similar multivariate model. Both multivariate analyses involved the ITT population.
Results
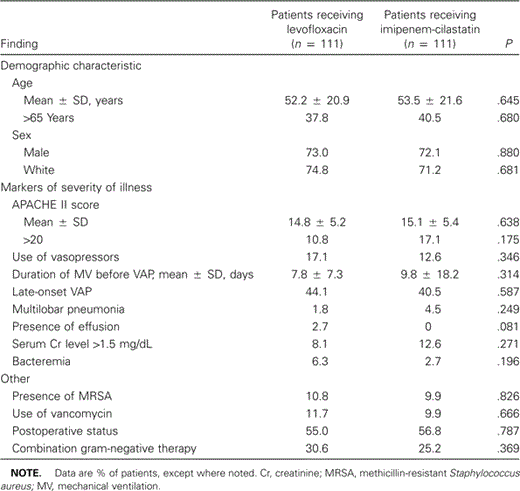
Of the 438 patients enrolled in the entire trial, 222 (50.7%) had VAP and comprised the ITT population. Exactly one-half of cohort with VAP received levofloxacin, and the other half received imipenem-cilastatin. As shown in table 1, the 2 treatment groups were similar with respect to a number of factors. The mean age of patients in each treatment arm was ∼53 years; nearly 4 of 10 patients with VAP were >65 years old. Most patients were men, and slightly more than half had developed their infection postoperatively. The duration of MV before the development of VAP was 7.8 α 7.3 days for patients receiving levofloxacin, compared with 9.8 α 18.2 days for patients receiving imipenem-cilastatin, but this variation was not statistically significant (P = .31). Slightly >40% of patients in both treatment groups had late-onset VAP.
Characteristics at baseline of patients with ventilator-associated pneumonia (VAP), by treatment group.
Severity of illness (table 1) did not vary by treatment group. The APACHE II score was 14.8 α 5.2 for patients receiving levofloxacin, compared with 15.1 α 5.4 for patients receiving imipenem-cilastatin. Although more patients receiving levofloxacin initially required vasopressors (17.1%), this difference was not statistically significant. Both multilobar infiltrates and pleural effusions were rare. Bacteremia was seen in 6.3% of patients receiving levofloxacin, compared with 2.7% of patients receiving imipenem-cilastatin.
After enrollment and randomization, patients received equivalent adjunct treatments (table 1). One of 3 patients receiving levofloxacin also received combination therapy for suspected or documented P. aeruginosa infection, compared with 1 of 4 patients receiving imipenem-cilastatin (P = .37). Likewise, investigators prescribed vancomycin for nearly 10% of patients, irrespective of the treatment arm. The duration of iv therapy was, on average, 1 day less for patients receiving levofloxacin (8.4 α 3.4 days) than for patients receiving imipenem-cilastatin (9.4 α 3.8 days) (P = .07).
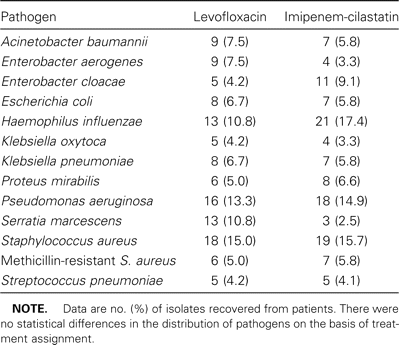
As table 2 shows, a wide spectrum of organisms was recovered. For patients receiving levofloxacin, the 3 most frequently isolated gram-negative pathogens were P. aeruginosa (n = 16), Serratia marcescens (n = 13), and Haemophilus influenzae (n = 13). For patients receiving imipenem-cilastatin, the most common gram-negative pathogens were H. influenzae (n = 21), P. aeruginosa (n = 18), and Enterobacter cloacae (n = 11). Acinetobacter baumannii was found in isolates from patients in both treatment arms (7.5% of isolates from patients receiving levofloxacin vs. 5.8% of isolates from patients receiving imipenem-cilastatin; P, not significant). More than 40% of all isolates were S. aureus and were evenly distributed between the 2 treatment arms. One-quarter of all S. aureus isolates demonstrated resistance to methicillin.
Distribution of respiratory pathogens, by treatment group, among patients with ventilator-associated pneumonia.
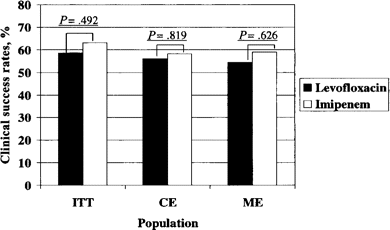
Figure 1 shows that there was no difference in rates of clinical success as a function of antibiotic therapy. Among the ITT cohort, 58.6% of patients receiving levofloxacin were classified as experiencing clinical success, compared with 63.1% of patients receiving imipenem-cilastatin (absolute difference, 4.5%; P = .49; 95% CI, -8.77–17.79%) More than half of the patients randomized were included in the clinically evaluable and the microbiologically evaluable cohorts. Clinical success rates were slightly lower among patients in the clinical evaluable and microbiologically evaluable cohorts, compared with the ITT cohort. However, antibiotic assignment did not appear to affect clinical success rates in these groups. In those patients infected with P. aeruginosa, clinical success rates were also comparable (87.5% for patients receiving levofloxacin vs. 61.1% for patients receiving imipenem-cilastatin; P, not significant). Most patients (>85%) survived. Patients receiving levofloxacin were 30% less likely to die than were patients receiving imipenem-cilastatin (OR, 0.70), but this difference was not statistically significant (95% CI, 0.33–1.48; P = .37).
Clinical success rates for the intent-to-treat (ITT), clinically evaluable (CE), and microbiologically evaluable (ME) populations of patients receiving levofloxacin or imipenem-cilastatin.
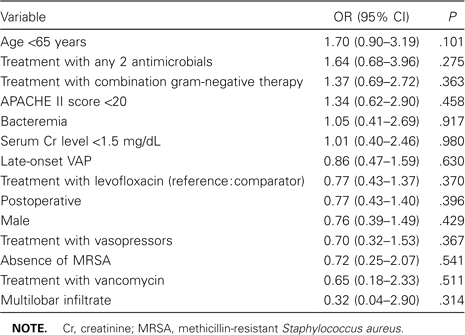
On the basis of the results of the univariate analysis, 3 variables in addition to treatment regimen (e.g., an APACHE II score of <20, presence of bacteremia, and treatment with levofloxacin) were included in an initial multivariate model focused on predictors of clinical success. None of these variables was independently statistically predictive of outcome. For example, having an APACHE score of <20 correlated with clinical success (OR, 1.52) but was not statistically significant (95% CI, 0.76–3.03; P = .234). In the second, expanded model, when all variables—including severity of illness, treatment with antibiotics other than study medications, and microbiological profile—were retained in the multivariate analysis, no factor significantly correlated with clinical results (table 3). In neither model did treatment regimen influence outcome, indicating that levofloxacin was equivalent to imipenem-cilastatin.
Multivariate predictors of clinical success in treating patients with ventilator-associated pneumonia (VAP).
Resistance emerged in 1 P. aeruginosa isolate in each arm (P, not significant). Superinfection with Pseudomonas species was more likely to occur in patients treated with imipenem-cilastatin (3 patients receiving levofloxacin vs. 10 patients receiving imipenem-cilastatin; P = .045)
SAEs were noted in 34 patients (30.6%) receiving levofloxacin and in 36 patients (32.4%) receiving imipenem-cilastatin (P, not significant). There was no difference in the distribution of types of SAEs as a function of antibiotic administered. The most common SAEs among patients receiving levofloxacin were aggravated condition (5.4%), respiratory insufficiency (4.5%), hypotension (3.6%), gastrointestinal hemorrhage (2.7%), sepsis (2.7%), and cerebrovascular disorder (2.7%). For patients receiving imipenem-cilastatin, the most common SAEs were respiratory insufficiency (11.7%), aggravated condition (3.6%), sepsis (3.6%), cardiac arrest (2.7%), pneumonia (2.7%), and acute renal failure (2.7%). The overall incidence of heart rate and rhythm disorders was similar (4.5%) for both treatment groups. SAEs leading to discontinuation of antimicrobials were also rare (4 patients receiving levofloxacin vs. 2 patients receiving imipenem-cilastatin).
Discussion
This secondary analysis of a large randomized trial reveals that levofloxacin performs as well as imipenem-cilastatin for treating VAP. This observation was seen in all 3 cohorts analyzed (i.e., the ITT, clinically evaluable, and microbiologically evaluable cohorts) and in patients with documented P. aeruginosa infection. Two separate multivariable models confirmed that this comparability does not appear to be a result of differences in the study populations at baseline.
Several other investigators have compared quinolones with imipenem-cilastatin for treating VAP. Fink et al. [10] compared ciprofloxacin (400 mg iv q8h) with imipenem-cilastatin for treating cases of severe pneumonia. Of the entire study sample of 405 patients, ∼60% had VAP. Fink et al. [10] did not report the incidence of late-onset VAP in their trial. Outcomes, which were assessed in a blinded fashion, were similar to those we noted in association with levofloxacin, in that 61% of patients receiving ciprofloxacin and 55% of patients receiving imipenem-cilastatin were categorized as having experienced clinical success. In multivariate analysis, Fink et al. [10] reported that there were 3 statistically significant predictors of clinical response: treatment with ciprofloxacin, absence of P. aeruginosa, and no requirement of MV. Unlike those authors, we did not note that any variable, including the investigational agent, correlated with clinical success. This discordance likely arises because of differences in the composition of the study populations. Moreover, we restricted our analysis to more severely ill patients who already required MV. On the other hand, Fink et al. [10] performed their analysis on their entire population of patients with pneumonia and included both patients who required MV and those who were breathing spontaneously.
In a smaller study, Torres et al. [11] randomized 152 patients with VAP to receive either ciprofloxacin or imipenem-cilastatin, and 75 of those patients were included in their efficacy analysis. P. aeruginosa was identified in one-third of cases. Similar to our study, the study of Torres et al. [11] used an open-label design. The patients in that trial appeared to be similar to those in the levofloxacin study, with respect to severity of illness as measured by APACHE II score. In contrast to both the study by Fink et al. [10] and the present study, Torres et al. [11] required microbiological confirmation of infection in all instances and precluded combination antimicrobial therapy. Torres et al. [11] noted slightly higher clinical success rates (∼75%), but this was not affected by treatment regimen. They concluded that ciprofloxacin and imipenem-cilastatin yielded “good clinical success rates” [11].
One important implication of these 3 trials is that coverage for anaerobic bacteria need not be included in empirical regimens for VAP. Although quinolones possess some activity against anaerobic bacteria, most are not consistently considered to be antianaerobic agents. Alternatively, carbapenems have excellent in vitro activity against anaerobic bacteria. The finding that empirical anaerobic coverage does not alter outcomes of VAP is consistent with the observations of Marik et al. [14]. In a prospective, cohort study of >140 patients with VAP, invasive bronchoscopic cultures yielded no anaerobic bacteria. Furthermore, Marik et al. [14] performed special cultures in an effort to isolate anaerobes, and, despite doing this, they still recovered no anaerobic bacteria. Empirical use of antianaerobic agents, although no more effective than quinolones for treating VAP, may contribute to the acquisition and spread of vancomycin-resistant enteroccoci in the ICU [15]. These observations, coupled with local drug-sensitivity patterns, need to be considered by clinicians when determining their initial antibiotic selections for VAP.
Until now, the most often used dose of levofloxacin was 500 mg q.d. For the present trial, the 750-mg dose was chosen to maximize the pharmacodynamic properties of levofloxacin. Since the activity of quinolones is concentration dependent, using a higher dose necessarily increases the maximum serum concentration of the agent, which, in turn, theoretically increases the likelihood of rapid killing and eradication of pathogens [9]. Likewise, more-rapid killing should help to limit potential emergence of resistance [9]. Added benefits of this daily-dosing approach are that it is more convenient and less expensive than regimens that necessitate more-frequent antimicrobial administration. Similarly, simpler dosing schedules also decrease the likelihoods that a dose will be missed and/or that therapy will be delayed.
Both superinfection with and emergence of resistance among P. aeruginosa in patients receiving levofloxacin were rare. This finding supports the aforementioned hypothesis regarding the implications of the pharmacodynamics of levofloxacin. Fink et al. [10] and Torres et al. [11] described the emergence of resistance in 50% and 33% of the imipenem-cilastatin-treated cohorts, respectively. In addition, resistance emerged in P. aeruginosa exposed to ciprofloxacin. Pooling the 2 ciprofloxacin groups from the studies of Fink et al. [10] and Torres et al. [11] demonstrates that resistance emerged in P. aeruginosa in nearly 1 in 4 (i.e., 14/61) patients.
Antibiotic resistance in general remains a major issue in the ICU. This is particularly true for P. aeruginosa. For example, >10% of P. aeruginosa isolates are now categorized as being resistant to carbapenems [16]. Neuhauser et al. [16] noted that, in the United States between 1994 and 2000, there was generally an absolute decrease of ⩽6% in the activity of most antimicrobials against gram-negative aerobic bacilli. However, during this period, the activity of ciprofloxacin decreased from 86% to 76% among Pseudomonas species [16]. This decrease in activity corresponded with an increase in the use of ciprofloxacin. Although Neuhauser et al. [16] did not examine resistance to levofloxacin, other investigators have found that ciprofloxacin and levofloxacin have similar susceptibility profiles for P. aeruginosa [17, 18]. Reliance on quinolones, therefore, must be couched in light of trends in resistance both nationally and locally. More important, an appreciation of local drug-resistance patterns allows physicians to achieve higher rates of initially appropriate antibiotic therapy, and quinolones have been demonstrated to be effective as part of such a strategy [19].
The present study has several limitations. First, it is technically retrospective since it represents a secondary analysis. However, unlike a traditional retrospective approach, we focused on prospectively collected information from a randomized trial, a fact that should necessarily limit bias. Second, although the 2 treatment groups appear to be comparable, there may be subtle differences between the populations that might confound our findings. To address this possibility, we performed several multivariate analyses to control for variations between the alternative therapeutic arms. Third, there are many factors other than antimicrobial selection that affect outcomes of VAP. We attempted to explore those factors by describing how patients were treated after randomization in terms of use of other antimicrobials, including both combination gram-negative agents and vancomycin. The fact that the trial was randomized should also help limit any variability, but this cannot be guaranteed. Fourth, despite this report describing one of the largest cohorts of patients with VAP, it still may have been too small to detect important clinical differences. On the basis of the clinical success rates we observed, we estimate that the study had only 15% power to detect a 20% difference in clinical success rates. Hence, any future trial adequately powered to explore these issues would be almost impossible to conduct because of sample size and cost concerns. Finally, as evidenced by the low mortality rate, the trial population may not be representative of typical cases of VAP in which mortality is reported to be higher. Nonetheless, the low frequency of death we observed is consistent with mortality rates noted in other randomized antibiotic investigations of VAP [10, 11].
In conclusion, the present analysis suggests that levofloxacin is effective for the treatment of VAP and is comparable to imipenem-cilastatin. Levofloxacin appears to be safe and well tolerated in patients with VAP. Levofloxacin represents a once-daily alternative for treatment of VAP.
Acknowledgments
Potential conflicts of interest. N.Z., S.-C.W., and A.M.T. were employees of Ortho-McNeil Pharmaceutical at the time the present study was conducted, but no extramural funding was provided for the preparation of this analysis. A.F.S. and M.H.K. have received research support from Ortho-McNeil Pharmaceutical, but no extra funding was provided for the preparation of this analysis. W.L.J. and A.S.R.: no conflicts.
References
The opinions expressed herein are not to be construed as official or as reflecting official policy of either the Department of the Army or the Department of Defense.





Comments