Summary
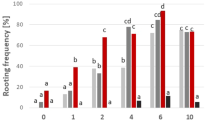
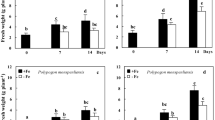
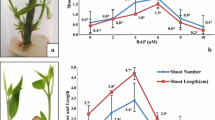
One easy-to-root and one difficult-to-root species of the ornamental plant Grevillea were investigated for rooting potential in relation to peroxidase activity. In vitro-grown shoot segments of both species started to root 30 d after transplanting to rooting medium containing indole-3-butyric acid (IBA), however, fewer roots were found on fewer segments of the difficult-to-root species G. petrophioides compared to the easy-to-root species G. rondeau. Total peroxidase (POX) activity was measured during the rooting process. G. petrophioides showed higher total POX activity at the time point of adventitious root formation than G. rondeau. Isoelectric focusing electrophoresis showed that G. rondeau contained more acidic isoforms than G. petrophioides, but the basic isoforms were more prominent in the difficult-to-root species, especially at the time point of lateral root emergence. In addition, the ability of different hormones to induced POX activity in upper and lower stem segments of both species was tested. Indole-3-acetic acid (IAA), IBA and α-naphthaleneacetic acid induced POX activity in the upper stem segments of G. rondeau, whereas the same hormones led to the induction of POX activity in the lower stem segments of G. petrophioides. Similar to the results obtained with Grevillea, the difficult-to-root variety of Protea showed higher POX activity, especially in the middle stem part and the leaves. Feeding of radiolabeled IAA to the Grevillea stem segments resulted in the synthesis of three different compounds in both species. After 1h incubation no differences were found in the uptake of IAA and the appearance of other labeled compounds. However, after 2 and 4 h incubation IAA uptake was faster in the easy-to-root species and IAA was also metabolized to a higher extent in G. rondeau. Three metabolites were found, tentatively identified as IAA-aspartate, IBA, and an IBA conjugate.
Similar content being viewed by others
References
Aeschbacher, R. A.; Schiefelbein, J. W.; Benfey, P. N. The genetic and molecular basis of root development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45:25–45; 1994.
Alvarez, R.; Nissen, S. J.; Sutter, E. G. Relationship between indole-3-acetic acid levels in apple (Malus pumila Mill) rootstocks cultured in vitro and adventitious root formation in the presence of indole-3-butyric acid. Plant Physiol. 89:439–443; 1989.
Baraldi, R.; Cohen, J. D.; Bertazza, D.; Predieri, S. Uptake and metabolism of indole-3-butyric acid during the in vitro rooting phase in pear cultivars (Pyrus communis L.). Acta Hort. 329:289–291; 1993.
Bartel, B.; LeClere, S.; Magidin, M.; Zolman, B. K. Inputs to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-butyric acid β-oxidation. J. Plant Growth Regul. 20:198–216; 2001.
Blazkova, A.; Sotta, B.; Tranvan, H.; Maldiney, R.; Bonnet, M.; Einhorn, J. H. Kerhoas, L.; Miginiac, E. Auxin metabolism and rooting in young and mature clones of Sequoia sempervivens. Physiol. Plant. 99:73–80; 1997.
Bruce, R.; West, C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 91:889–897; 1989.
Chao, I.-L.; Cho, C.-L.; Chen, L.-M.; Liu, Z.-H. Effect of indole-3-butyric acid on the endogenous indole-3-acetic acid and lignin contents in soybean hypocotyl during adventitious root formation. J. Plant Physiol. 158:1257–1262; 2001.
Cooper, W. C. Hormones in relation to root formation on stem cuttings. Plant Physiol. 10:789–794; 1935.
Davies, F. T., Jr.; Davis, T. D.; Kester, D. E. Commercial importance of adventitious rooting to horticulture. In: Davis, T. D.; Haissig, B. E., eds, Biology of adventitious root formation. New York: Plenum Press; 1994;53–59.
Epstein, E.; Ludwig-Müller, J. Indole-3-butyric acid in plants: occurrence, biosynthesis, metabolism, and transport. Physiol. Plant. 88:382–389; 1993.
Epstein, E.; Zilkah, S.; Faingersh, G.; Rotebaum, A. Transport and metabolism of indole-3-butyric acid in sterile easy-to-root and difficult-to-root cuttings of sweet cherry (Prunus avium L.). Acta Hort. 329:292–295; 1993.
Flott, B. E.; Moerschbacher, B. M.; Reisener, H. J. Peroxidase isoenzyme patterns of resistant and susceptible wheat leaves following stem rust infection. New Phytol. 111:413–421; 1989.
Friedman, R.; Altman, A.; Zamski, E. Advantitious root formation in bean hypocotyl cuttings in relation to IAA translocation and hypocotyls anatomy. J. Exp. Bot. 30:768–777; 1979.
Fukuda, H.; Komamine, A. Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta 115:423–430; 1982.
Gaspar, T.; Kevers, C.; Hausman, J. F.; Berthon, J. Y.; Ripetti, V. Practical use of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 12:757–765; 1992.
Gotthardt, U.; Grambow, H. J. Near-isogenic wheat suspension cultures: establishment, elicitor induced peroxidase activity and potential use in the study of host/pathogen-interactions. J. Plant Physiol. 139:659–665; 1992.
Harbage, J. F.; Stimart, D. P. Effect of pH and 1H-indole-3-butyric acid (IBA) on rooting of apple microcuttings. J. Am. Soc. Hort. Sci. 121:1049–1053; 1996.
Hartmann, H. T.; Kester, D. E.; Davies, F. T. Plant propagation: principles and practices. Englewood Cliffs, NJ: Prentice-Hall; 1990:246–247.
Ishige, F.; Mori, H.; Yamazaki, K.; Imaseki, H. Identification of a basic glycoprotein induced by ethylene in primary leaves of azuki bean as a cationic peroxidase. Plant Physiol. 101:193–199; 1993.
Joersbo, M.; Andersen, J. M.; Okkels, F. T.; Rajagopal, R. Isoperoxidases as markers of somatic embryogenesis in carrot cell suspension cultures. Physiol. Plant. 76:10–16; 1989.
Kerby, K.; Somerville, S. C. Purification of an infection-related, extracellular peroxidase from barley. Plant Physiol. 100:397–402; 1992.
Klotz, K. L.; Lagrimini, L. M. Phytohormone control of the tobacco anionic peroxidase promoter. Plant Mol. Biol. 31:565–574; 1996.
Kovar, J. L.; Kuchenbuch, R. O. Commercial importance of adventitious rooting to agronomy. In: Davis, T. D.; Haissig, B. E., eds. Biology of adventitious root formation. New York: Plenum Press; 1994:25–34.
Lagrimini, L. M. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 96:577–583; 1991.
Lagrimini, L. M.; Rothstein, S. Tissue specificity of tobacco peroxidase isoenzymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiol. 84:438–442; 1987.
Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 32:219–230; 2000.
Ludwig-Müller, J.; Epstein, E. Occurrence and in vivo biosynthesis of indole-3-butyric acid in corn (Zea mays L.). Plant Physiol. 97:765–770; 1991.
Ludwig-Müller, J.; Epstein, E. Indole-3-butyric acid in Arabidopsis thaliana. II. In vivo metabolism. Plant Growth Regul. 13:189–195; 1993.
Ludwig-Müller, J.; Epstein, E. Indole-3-butyric acid in Arabidopsis thaliana. III. In vivo biosynthesis. Plant Growth Regul. 14:7–14; 1994.
Ludwig-Müller, J.; Hilgenberg, W. Tryptophan oxidizing enzyme and basic peroxidase isoenzymes in Arabidopsis thaliana (L.) Heynh: are they identical? Plant Cell Physiol. 33:1115–1125; 1992.
Ludwig-Müller, J.; Hilgenberg, W. Characterization and partial purification of indole-3-butyric acid synthetase from maize (Zea mays). Physiol. Plant. 94:651–660; 1995.
Ludwig-Müller, J.; Hilgenberg, W.; Epstein, E. The in vitro biosynthesis of indole-3-butyric acid in maize. Phytochemistry 40:61–68; 1995a.
Ludwig-Müller, J.; Kaldorf, M.; Sutter, E. G.; Epstein, E. Indole-3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus intraradices. Plant Sci. 125:153–162; 1997.
Ludwig-Müller, J.; Schubert, B.; Pieper, K. Regulation of IBA synthetase by drought stress and abscisic acid. J. Exp. Bot. 46:423–432; 1995b.
Ludwig-Müller, J.; Thermann, P.; Pieper, K.; Hilgenberg, W. Peroxidase and chitinase isoenzyme activities during root infection of Chinese cabbage with Plasmodiophora brassicae Woron. Physiol. Plant. 90:661–670; 1994.
Ludwig-Müller, J.; Town, C. D. Molecular and genetic studies of adventitious root formation in Arabidopsis. Abstract No. 287, 15th Int. Conf. on Plant Growth Substances, Minneapolis; 1995.
Marchant, A.; Bhalearo, R.; Casimiro, I.; Eklöf, J.; Casero, P. J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597; 2002.
Markkola, A. M.; Ohtonen, R.; Tarvainen, O. Peroxidase activity as an indicator of pollution stress in the fine roots of Pinus sylvestris. Water Air Soil Pollut. 52:149–156; 1990.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Nakajima, R.; Yamazaki, I. The mechanism of indole-3-acetic acid oxidation by horseradish peroxidase. J. Biol. Chem. 254:872–878; 1979.
Nordström, A.-C.; Jacobs, F. A.; Eliasson, L. Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol. 96:856–861; 1991.
Olde, P.; Marriott, N. The Grevillea book, vols. 1–3. Kenthurst, NSW: Kangaroo Press, 1994.
Paterson, J. C. The Protea family in Southern Africa. Cape Town: Struik; 2000.
Pressey, R. Anions activate the oxidation of indoleacetic acid by peroxidases from tomato and other sources. Plant Physiol. 93:798–804; 1990.
Riov, J.; Yang, S. F. Ethylene and auxin-ethylene interaction in adventitious root formation in mung bean (Vigna radiata) cuttings. J. Plant Growth Regul. 8:131–141; 1989.
Sato, Y.; Sugiyama, M.; Gorecki, R. J.; Fukuda, H.; Komamine, A. Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia. Planta 189:584–589; 1993.
Schrauwen, J. Nachweis von Enzymen nach elektrophoretischer Trennung an Polyacrylamidsäuren. J. Chromatogr. 23:177–180; 1966.
van der Krieken, W. M.; Breteler, H.; Visser, M. H. M. The effect of conversion of indolebutyric acid into indoleacetic acid on root formation. Plant Cell Physiol. 33:709–713; 1992.
van der Krieken, W. M.; Breteler, H.; Visser, M. H. M.; Mavridou, D. The role of the conversion of IBA into IAA on root generation in apple: introduction of a test system. Plant Cell Rep. 12:203–206; 1993.
Wiesman, Z.; Riov, J.; Epstein, E. Characterization and rooting ability of indole-3-butyric acid conjugates formed during rooting of mung bean cuttings. Plant Physiol. 91:1080–1084; 1989.
Zimmerman, P.W.; Wilcoxon, F. Several chemical growth substances which cause initiation of roots and other responses in plants. Contrib. Boyce Thompson Inst. 7:209–229; 1935.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludwig-Müller, J. Peroxidase isoenzymes as markers for the rooting ability of easy-to-root and difficult-to-root Grevillea species and cultivars of Protea obstusifolia (Proteaceae). In Vitro Cell.Dev.Biol.-Plant 39, 377–383 (2003). https://doi.org/10.1079/IVP2003423
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003423