Summary
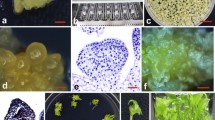
An efficient regeneration and transformation system was developed for two elite aspen hybrid clones (Populus canescens × P. grandidentada and P. tremuloides × P. davidiana). Callus was induced from in vitro leaf explants on modified Murashige and Skoog medium (MSA) and woody plant medium (WPM) containing four different combinations of cytokinins and auxins. Callus tissues regenerated into shoots on WPM medium supplemented with 2.0 mgl−1 (9.12 μM) zeatin or 0.01 mgl−1 (0.045 μM) thidiazuron. P. canescens × P. grandidentata exhibited the higher callus and shoot production. In vitro leaf explants from the two hybrid clones were cocultivated with Agrobacterium tumefaciens strain EHA105 harboring the binary Ti plasmid pBI121 carrying the uidA gene encoding for β-glucuronidase (GUS) and the npt II gene encoding for neomycin phosphotransferase II. Transformation was confirmed by GUS assays, polymerase chain reaction, and Southern blot analyses. Agrobacterium concentration, acetosyringone, and pH of the cocultivation medium were evaluated for enhancing transformation efficiency with the clone P. canescens × P. grandidentata.
Similar content being viewed by others
References
Blake, N. K.; Ditterline, R. L.; Stout, R. G.; Polymerase chain reaction used for monitoring multiple gene integration in Agrobacterium-mediated transformation. Crop. Sci. 31:1686–1688; 1991.
Cheng, Z.-M.; Schnurr, J. P. Regeneration and transformation of elite aspen hybrids In: Program and Abstracts, International poplar symposium, poplar biology and its implications for management and conservation. University of Washington, Seattle, August 20–25; 1995:40.
Confalonieri, M.; Balestrazzi, A.; Bisoffi, S.; Cella, R. Factors affecting Agrobacterium tumefaciens-mediated transformation in several black poplar clones. Plant Cell Tiss. Organ Cult. 43:215–222; 1995.
De Block M., Factors influencing the tissue culture and the Agrobacterium tumefaciens-mediated transformation of hybrid aspen and poplar clones. Plant Physiol. 93:1110–1116; 1990.
Dellaporta, S. L.; Wood, J.; Hicks, J. B. A plant DNA mini-preparation: version II. Plant Mol. Biol. Rep. 1:19–21; 1983.
Dickmann, D. I.; Stuart, K. W. The culture of poplars in eastern North America. Ann Arbor, MI: McNaughton & Gunn, Inc.; 1983:168.
Douglas, G. C. Poplar (Populus spp.). In: Bajaj, Y. P. S., ed. Biotechnology in agriculture and forestry, Vol. 5. New York: Springer-Verlag; 1989:299–323.
Eckenwalder, J. E. Systematics and evolution of Populus. In: Stettler, R. F.; Bradshaw, H. D. Jr.; Heilman, P.; Hinckley, T. M, eds. Biology of Populus and its implications for management and conservation. Ottawa NRC Research Press, National Research Council of Canada; 1996:7–32.
Fillatti, J. J.; Sellmer, J.; McCown, B.; Haissig, B.; Comai, L. Agrobacterium-mediated transformation and regeneration of Populus. Mol. Gen. Genet. 206:192–199; 1987.
Godwin, I.; Gordon, T.; Ford-Lloyd, B.; Newbury, H. J. The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep. 9:671–675; 1991.
Graham, R. L. An analysis of the potential land base for energy crops in the conterminous states. Biomass Bioenergy 6:175–179; 1994.
Han, K.-H.; Gordon, M. P.; Strauss, S. H. Cellular and molecular biology of Agrobacterium-mediated transformation of Populus. In: Stettler, R. F.; Bradshaw, H. D. Jr.; Heilman, P.; Hinckley, T. M., eds. Biology of Populus and its implications for management and conservation. Ottawa. NRC Research Press, National Research Council of Canada; 1996:201–222.
Han, K.-H.; Meilan, R.; Ma, C.; Strauss, S. H. An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus). Plant Cell Rep. 19:315–320; 2000.
Hood, E. E.; Gelvin, S. B.; Melchers, L. S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 2:208–218; 1993.
Jefferson, R. A. Assaying chimeric genes in plant—the GUS gene fusion system. Plant Mol. Biol. Rep. 5:387–405; 1987.
Jouanin, L.; Brasilerio, C. M.; Leple, J. C.; Pilate, G.; Cornu, D. Genetic transformation: a short review of methods and their applications, results and perspectives for forest trees. Ann. Sci. For. 50:325–336; 1993.
Klopfenstein, N. B.; Shi, N.-Q.; Kernan, A.; Mcnabb, H. S. Jr.; Hall, R. B.; Hart, E. R.; Thornburg, R. W. Transgenic Populus hybrid expresses a wound-inducible potato proteinase inhibitor II-CAT gene fusion. Can. J. For. Res. 21:1321–1328; 1991.
Li, B.; Wyckoff, G. W.; Einspahr, D. W. Hybrid aspen performance and genetic gains. Northern J. Appl. For. 10:117–122; 1993.
Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurelo, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Prop. Soc. 30:421–427; 1980.
Mathews, H.; Bharathan, N.; Litz, R. E.; Narayanan, K. R.; Rao, P. S.; Bhatia, C. R. The promotion of Agrobacterium mediated transformation in Atropa belladonna L. by acetosyringone. J. Plant Physiol. 136:404–409; 1990.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Pâques, M.; Boxus, P. Vitrification: review of literature. Acta Hort. 212:156–166; 1987.
Porebski, L.; Bailey, L.; Baum, B. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15:8–15; 1997.
SASI. SAS user's guide: statistics. Cary, NC; Statistical Analysis System Institute; 1990.
Sheikholeslam, S. N.; Weeks, D. P. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol. Biol. 8:291–298; 1987.
Stachel, S. E.; Messens, E.; Van Montagu, M.; Zambryski, P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629; 1985.
Tsai, C.-J.; Podilia, G. K.; Chiang, V. L. Agrobacterium-mediated transformation of quaking aspen (Populus tremuloides) and regeneration of transgenic plants. Plant Cell Rep. 14:94–97; 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dai, W., Cheng, ZM. & Sargent, W. Plant regeneration and Agrobacterium-mediated transformation of two elite aspen hybrid clones from in vitro leaf tissues. In Vitro Cell.Dev.Biol.-Plant 39, 6–11 (2003). https://doi.org/10.1079/IVP2002355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2002355