Abstract
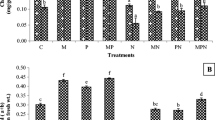
The effects of antagonistic fungi (Paecilomyces lilacinus, Pochonia chlamydosporia and Trichoderma harzianum) and plant growth-promoting rhizobacteria (PGPR), namely Bacillus subtilis, Paenibacillus polymyxa and Burkholderia cepacia, were studied alone and in combination in glasshouse experiments on the growth of tomato and on the reproduction of the nematode Meloidogyne incognita. Application of antagonistic fungi and PGPR caused a significant (P < 0.05) increase in tomato growth (based on shoot dry weight) both with and without nematodes. P. lilacinus was more effective in reducing galling and improving the growth of nematode-inoculated plants than T. harzianum, while P. polymyxa was more effective than B. subtilis. The greatest increase in growth of nematode-inoculated plants and reduction in nematode galling was observed when P. polymyxa was used with P. lilacinus or P. chlamydosporia. P. lilacinus parasitised more females and eggs than the other fungi tested. Root colonisation by P. polymyxa was high when used alone but reduced in the presence of antagonistic fungi.
Similar content being viewed by others
References
Bevivino A, Sarrocco S, Dalmastri C, Tabacchioni S, Cantale C, Chiarini L (1998) Characterization of a free-living maize-rhizosphere population of Burkholderia cepacia: effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiology Ecology 27, 225–237. doi: 10.1111/j.1574-6941.1998.tb00539.x
Bhatti DS, Jain RK (1977) Estimation of loss in okra, tomato and brinjal yield due to Meloidogyne incognita. Indian Journal of Nematology 7, 37–41.
Bourne JM, Kerry BR (1998) Effect of the host plant on the efficacy of Verticillium chlamydosporium as a biological control agent of root-knot nematodes at different nematode densities and fungal application rates. Soil Biology & Biochemistry 31, 75–84. doi: 10.1016/S0038-0717(98) 00107-2
Broadbent P, Baker KFM, Franks N, Holland J (1977) Effect of Bacillus sp. on increased growth of seedlings in steamed and non-treated soil. Phytopathology 67, 1027–1034.
Chiarini L, Bevivino A, Tabacchioni S, Dalmastri C (1998) Inoculation of Burkholderia cepacia, Pseudomonas fluorescens and Enterobacter sp. on Sorghum bicolor: root colonization and plant growth promotion of dual strain inocula. Soil Biology & Biochemistry 30, 81–87. doi: 10.1016/ S0038-0717(97)00096-5
Daubaras DL, Danganan CE, Hubner A, Ye RW, Hendrickson W, Chakrabarty AM (1996) Biodegradation of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia strain AC1100: evolutionary insight. Gene 179, 1–8. doi: 10.1016/S0378-1119(96) 00326-5
De Freitas JR, Banerjee MR, Germida JJ (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biology and Fertility of Soils 24, 358–364. doi: 10.1007/s003740050258
Egamberdiyeva D, Höflich G (2004) Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. Journal of Arid Environments 56, 293–301. doi: 10.1016/S0140-1963(03)00050-8
Glick BR (1995) The enhancement of plant growth by free living bacteria. Canadian Journal of Microbiology 41, 109–117.
Gokte N, Swarup G (1988) On the potential of some bacterial biocides against root-knot and cyst nematodes. Indian Journal of Nematology 18, 152–153.
Jagdale GB, Grewal PS (2002) Identification of alternatives for the management of foliar nematodes in floriculture. Pest Management Science 58, 451–458. doi: 10.1002/ps.472
Jatala P (1986) Biological control of plant parasitic nematodes. Annual Review of Phytopathology 24, 453–489. doi: 10.1146/annurev.py.24. 090186.002321
Kerry BR (2000) Rhizosphere interactions and the exploitation of microbial agents for the biological control of nematodes. Annual Review of Phytopathology 38, 423–441. doi: 10.1146/annurev.phyto.38.1.423
Kokalis-Burelle N, Vavarina CS, Rosskopf EN, Shelby RA (2002) Field evaluation of plant growth promoting rhizbacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant and Soil 238, 257–266. doi: 10.1023/A:1014464716261
Lewis JA, Papavizas GC (1980) Integrated control of Rhizoctonia fruit rot of cucumber. Phytopathology 70, 85–89.
Merriman PR, Prince RD, Kollmorgen JF, Piggott T, Ridge EH (1974) Effect of seed inoculation with Bacillus subtilis and Streptomyces griseus on the growth of cereals and carrots. Australian Journal of Agricultural Research 25, 219–226. doi: 10.1071/AR9740219
Mueller JG, Devereux R, Santavy DL, Lantz SE, Willis SG, Pritchard PH (1997) Phylogenetic and physiological compartment of PAH-degrading bacteria from geographically diverse soils. Antonie Van Leeuwenhoek 71, 329–343. doi: 10.1023/A:1000277008064
Nelson LM (2004) Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants. Crop Management. doi: 101094/Cm-2004-0301-05-RV
Oedjijono M, Line A, Dragar C (1993) Isolation of bacteria antagonistic to a range of plant pathogenic fungi. Soil Biology & Biochemistry 25, 247–250. doi: 10.1016/0038-0717(93)90034-9
Papavizas GC (1985) Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Annual Review of Phytopathology 23, 23–54. doi: 10.1146/annurev.py.23.090185.000323
Reddy DDR (1985) Analysis of crop losses in tomato due to Meloidogyne incognita. Indian Journal of Nematology 15, 55–59.
Riker AJ, Riker RS (1936) ‘Introduction of research of plant diseases.’ (John’s Swift Co. Inc.: St Louis, MO)
Sharma PD (2001) ‘Microbiology.’ (Rastogi Publications: Meerut, India)
Siddiqui ZA (2006) PGPR: prospective biocontrol agents of plant pathogens. In ‘PGPR: biocontrol and biofertilization.’ (Ed. ZA Siddiqui) pp. 111–142. (Springer: Dordrecht, The Netherlands)
Siddiqui ZA, Mahmood I (1993) Biological control of Meloidogyne incognita race-3 and Macrophomina phaseolina by Paecilomyces lilacinus and Bacillus subtilis alone and in combination of chickpea. Fundamental and Applied Nematology 16, 215–218.
Siddiqui ZA, Mahmood I (1995) Biological control of Heterodera cajani and Fusarium udum by Bacillus subtilis, Bradyrhizobium japonicum and Glomus fasciculatum on pigeon pea. Fundamental and Applied Nematology 18, 559–566.
Siddiqui ZA, Mahmood I (1996a) Biological control of plant parasitic nematodes by fungi: a review. Bioresource Technology 58, 229–239. doi: 10.1016/S0960-8524(96)00122-8
Siddiqui ZA, Mahmood I (1996b) Biological control of Heterodera cajani and Fusarium udum by Glomus mosseae, Trichoderma harzianum and Verticillium chlamydosporium on pigeon pea. Israel Journal of Plant Sciences 44, 49–56.
Sidhu AS (1998) Current status of vegetable research in India. In ‘World Conference on Horticulture Research, Rome, Italy’. Available at http:// www.agrsci.unibo.it/wchr/wc2/asv.html[Verified 10 September 2008].
Southey JF (1986) ‘Laboratory method for work with plant and soil nematodes.’ (Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office: London)
Tabacchioni S, Bevivino A, Chiarini L, Visca P, Del Gallo M (1993) Characteristics of two rhizosphere isolates of Pseudomonas cepacia and their potential plant-growth-promoting activity. Microbial Relations 2, 161–168.
Timmusk S, Nicander B, Granhall U, Tillberg E (1999) Cytokinin production by Paenibacillus polymyxa. Soil Biology & Biochemistry 31, 1847–1852. doi: 10.1016/S0038-0717(99)00113-3
Turner JT, Backman PA (1986) Biological cultures test control. Plant Disease 1, 49.
Verdejo-Lucas S, Sorribas FJ, Ornat C, Galeano M (2003) Evaluating Pochonia chlamydosporia in a double-cropping system of lettuce and tomato in plastic houses infested with Meloidogyne javanica. Plant Pathology 52, 521–528. doi: 10.1046/j.1365-3059.2003.00873.x
Viaene NM, Abawi GS (2000) Hirsutella rhosiliensis and Verticillium chlamydosporium as biological control agents of the root-knot nematode Meloidogyne hapla on lettuce. Journal of Nematology 32, 85–100.
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annual Review of Phytopathology 26, 379–407. doi: 10.1146/annurev.py.26.090188.002115
Wilson M, Backman PA (1999) Biological control of plant pathogens. In ‘Handbook of pest management.’ (Ed. JR Ruberson) pp. 309–335. (Marcel Dekker, Inc.: New York)
Windham GL, Windham MT, Williams WP (1989) Effects of Trichoderma spp. on maize growth and Meloidogyne arenaria reproduction. Plant Disease 73, 493–494. doi: 10.1094/PD-73-0493
Yuen GY, Schroth MN, McCain AH (1985) Reduction in Fusarium wilt of carnation with suppressive soils and antagonistic bacteria. Plant Disease 69, 1071–1075. doi: 10.1094/PD-69-1071
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqui, Z.A., Akhtar, M.S. Effects of antagonistic fungi and plant growth-promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita . Australasian Plant Pathology 38, 22–28 (2009). https://doi.org/10.1071/AP08072
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP08072