A miniature dosage meter for toxic gas is developed based on TaS2 nanosheets, which is capable of indicating the toxic dosage of trace level NO at room temperature. The TaS2 film-based chemiresistor shows an irreversible current response against the exposure of NO. The unique non-recovery characteristic makes the TaS2 film-based device an ideal indicator of total dosage of chronicle exposure.
The chemical exposure, especially toxic gases, in both working and living environment is gaining increasing public attentions from both government regulation and individual awareness. Although the conventional electronic gas sensors based on semiconducting metal oxide achieved huge success in the past decade,1 their work in high-temperature region requires huge power consumption.2,3 The recent research aims at low-cost, portable, and real-time gas sensors that are suitable for field-test. The incorporation of the emerging nanomaterials for electronic gas sensors,4–6 not only showed extreme sensitivity, but also opened the possibility to portable gas sensors that operate at room temperature. For example, one-dimensional (1D) nanowires and nanotubes7–9 and two-dimensional (2D) nanosheets10–13 are being studied intensively for the next generation gas sensors. Among those, the thin-film devices based on 1D/2D nanomaterials are promising candidates, owing to their scalable production and excellent reproducibility.
NOx is one of the most prominent air pollutants from the combustion of fossil fuels. The toxic effect of low-level NOx exposure is often transient and recoverable. However, chronicle exposure to low-level NOx still poses threat to human health.14,15 A gas sensor that can indicate the total dosage of low concentration exposure is therefore desired. For example, the European Union (EU) emission legislation required the hourly dosage of NOx in air to be less than 200 mg m−3. This is equal to an hourly exposure to a mean concentration of 0.1 ppm. This concentration is far above the detection limit of the most recently reported gas sensors based on nanomaterials.12,16,17 However, the long recovery time of these sensors due to the strong physical adsorption of NOx poses challenges in integrating the long-term dosage in low concentration. In addition, the often non-linear relation12,13 between the current response and analyte concentration makes it even challenging to determine the total dosage in concentration fluctuation. The most recent development of metal oxide sensors employed the gas-accumulable materials18,19 with much high adsorption rate than the desorption rate towards toxic gas. However, these semiconductor-based sensors all operate at above 300 °C which require large power consumption, and it is impossible for the portable detection.
In this communication, we present a new type of toxic gas sensor that can detect the chronicle dosage of nitric oxide (NO) at sub-ppm level. The uniform TaS2 film made from filtration of the homogenous suspension of single-to-few layer TaS2 nanosheets was used as the active layer for the chemiresistor sensor. The device showed an irreversible current response against the NO exposure. In addition, no current recovery was observed even after long time purge with N2 or heating the sample. The TaS2 film-based sensor showed linear current response in very low concentration and responded quickly to the change of the concentration. These properties make it possible to detect the total dosage of NO under fluctuating concentration. Our dosage meter promises a low-cost, miniature, and portable solution for constant monitoring the chronicle exposure of toxic gases.
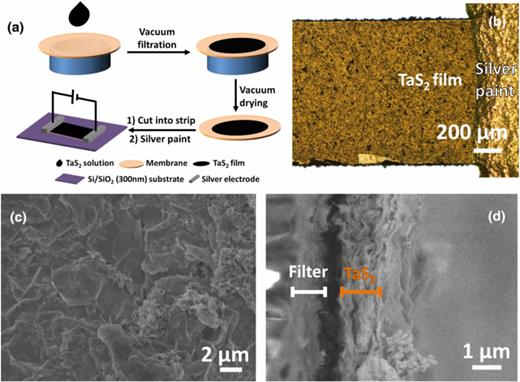
The TaS2 thin film is prepared via the vacuum filtration of TaS2 aqueous suspension obtained from the electrochemical lithium-intercalation method described in our previous reports.20–22 In this study, the single- to few-layer TaS2 nanosheets were used, which were confirmed by the transmission electron microscopy and atomic force microscopy.21 Fig. 1(a) shows the schematic illustration of the device fabrication. The TaS2 solution was purified by centrifugation to remove the thick and fragmental TaS2 sheets, before it was filtrated through a nitrocellulose membrane filter (25 mm in diameter, 0.8 mm pore size; Millipore, USA). The film was then dried in vacuum oven at room temperature for 12 h. The thickness of TaS2 film was controlled by adjusting the volume of the suspension. This filtration method is capable of producing uniform film over large area with suspension of nanomaterials such as graphene23 and carbon nanotube.24 The obtained TaS2 film was cut into 3 × 1 mm2 rectangular strip used as active channel without the removal of the underneath filter. The polymer membrane filter, therefore, acts as the supporting substrate for the TaS2 film to enhance the mechanical property. Such relatively big channel size was chosen to simplify the device fabrication and avoid the possible contamination from lithography process. Planar chemiresistor sensor was fabricated by placing the rectangular TaS2 strip onto Si/SiO2 (300 nm) substrate, followed by deposition of silver paint on both ends of the rectangular strip as the drain and source electrodes. More importantly, this preparation method gives an excellent reproducibility owing to the controllable filtration method to form uniform film, and the facile process to produce silver paint-based electrode contacts. Fig. 1(b) shows the optical image of a typical silver contact on TaS2 thin film channel with channel width of 1 mm. Scanning electron microscopy (SEM, JSM-7600F, JEOL Ltd., Tokyo) image shows the obtained TaS2 film on the filter (Fig. 1(c)). The thickness of the film is controlled by the volume of TaS2 solution used in vacuum filtration. Fig. 1(d) shows a typical cross-sectional SEM image of the obtained TaS2 film with a thickness of 1.3 μm.
(a) Schematic illustration of the fabrication of TaS2 device by vacuum filtration. (b) The optical image of the interface of TaS2 channel and silver electrode. SEM topography image (c) and cross-sectional image (d) of TaS2 film.
(a) Schematic illustration of the fabrication of TaS2 device by vacuum filtration. (b) The optical image of the interface of TaS2 channel and silver electrode. SEM topography image (c) and cross-sectional image (d) of TaS2 film.
The performance of the fabricated TaS2 gas sensor was evaluated by its current profile upon exposure to the NO gas with controlled concentration at a bias of 0.4 V at room temperature. All the gas sensing experiments were performed in the chamber of a homemade sensing system. The Keithley 4200 semiconductor characterization system was used to monitor the real-time current change using the two-point measurement. The concentration of NO flow, measured by the mass flow meter, was controlled by a mixture of NO (4.9 ppm in N2) and N2 (99.999%) flow (National Oxygen Pte Ltd, Singapore). The different ratio of NO to N2, i.e., 0:5, 0.2:4.8, 1:4, 2:3, 2.5:2.5, 5:0, was tested to give final NO concentration of 0 ppm, 0.20 ppm, 0.98 ppm, 1.96 ppm, 2.45 ppm, 4.9 ppm, respectively. As a proof of concept, the TaS2 film with thickness of 1.3 μm was used in the following test.
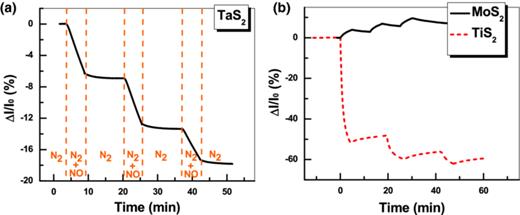
As shown in Fig. 2(a), a decrease of current was observed upon exposure of TaS2 film-based sensor to NO. Surprisingly, the TaS2 sensor shows no current recovery process after stopping NO and then purging with N2. Such irreversible current response was rarely observed in electronic gas sensors based on other nanomaterials.17,25 One typical example of a similar irreversible current response was the H2S sensor based on gold nanowire modified carbon nanotube.25 The reason for their irreversible response against H2S was explained by the strong affinity of gold to sulfur. Their current can easily be recovered by heating the device at above 140 °C, which is a common recovery method in gold-based H2S sensor.26 However, no current recovery was observed in our TaS2 based devices after long-time purging of N2 or heating of the device, indicating a different nature of the current decrease. This completely irreversible response has never been observed in other chemiresistor or field-effect transistor gas sensors based on nanomaterials.10,11 As control experiments, devices based on MoS2 and TiS2 nanosheets obtained by our electrochemical lithium-intercalation method22 were tested to compare their gas sensing behavior. Fig. 2(b) shows the typical current responses of devices based on MoS2 and TiS2 thin films prepared by vacuum filtration of their aqueous suspensions. The current recovery was observed in both devices, indicating a clear desorption process of NO gas after purge with N2. For the TaS2 thin film, the lack of current recovery may be due to an irreversible adsorption of the NO gas molecules on TaS2 nanosheets.
The typical current response of gas sensors upon the exposure of 1.96 ppm NO at room temperature with N2 as purge gas. The channel materials are (a) TaS2 film, and (b) MoS2 and TiS2 films prepared by the similar method for TaS2 film.
The typical current response of gas sensors upon the exposure of 1.96 ppm NO at room temperature with N2 as purge gas. The channel materials are (a) TaS2 film, and (b) MoS2 and TiS2 films prepared by the similar method for TaS2 film.
Fig. 3(a) shows the current responses of TaS2 devices against NO exposure at different concentrations for 10 min. A quasi-linear relation at low concentration between the current response and the NO concentration is found at ppm level, as shown in Fig. 3(b). Such linear current profile is essential in realizing the integration of the total dosage of NO at fluctuating concentration.
(a) The time-dependent current response of TaS2 sensors at different concentration of NO. (b) Plot of the detection sensitivity versus the concentration of NO. Standard error was obtained by testing 5 devices from the same batch of TaS2 film. (c) Current profile of device under continuous exposure of NO. The dotted red lines in (c) show the concentration profile of exposed NO. (d) Plot of the current response versus the integrated toxic load.
(a) The time-dependent current response of TaS2 sensors at different concentration of NO. (b) Plot of the detection sensitivity versus the concentration of NO. Standard error was obtained by testing 5 devices from the same batch of TaS2 film. (c) Current profile of device under continuous exposure of NO. The dotted red lines in (c) show the concentration profile of exposed NO. (d) Plot of the current response versus the integrated toxic load.
In order to further prove the concept of dosage meter, the current responses of a typical TaS2 device upon continuous exposure of NO at 5 different concentrations were tested (Fig. 3(c)). The sensitivity, i.e., rate of current decrease, increased with the NO concentration increased from 0.19 ppm to 4.9 ppm. To further investigate the relation between the current profile and the total dosage of NO exposure, the concept of toxic load (L)27 was used to quantify the dosage of hazardous gas. Although the theoretical model of effective toxicity of toxic gas with fluctuating concentrations is under debate for decades,28,29 this study follows the modified Haber's Rule30 to quantify the total toxic load for its simplicity. The modified Haber's rule defines the toxic load as
where C is the concentration of toxic gas, t is the exposure time, and n is a constant for the specific gas from experimental data. The unit used for L is ppm min. Since n = 1 in the case of NO given by Health and Safety Executive, a linear relation between NO concentration and current response in the same exposure period, as shown in Fig. 3(b), meets the requirement for a dosage meter. Fig. 3(d) shows the linear relation between the current response and the calculated total toxic load for our TaS2 device. Therefore, our TaS2-based device meets the requirements for a dosage meter, in which the total toxic load can be directly read out from the current profile.
An exponential current profile over long-time exposure is observed, as shown in Fig. 4(a). The detection range of the dose meter was characterized by the continuous exposure of 4.9 ppm NO gas until 1/e (mean lifetime) of its initial current was reached. The conductance of device (thickness of 1.3 μm) decreased 63% (1-1/e) after the continuous exposure to 750 ppm min of NO, as shown in the blue line in Fig. 4(a). As a comparison, the dosage meter based on recent development metal oxide sensor has a maximum dosage range below 100 ppm min.18 In addition, the relation between the detection range and the film thickness was also investigated. TaS2 film with different thickness was fabricated by increasing the solution volume of TaS2 suspension with the same concentration in filtration. TaS2 films with 3 different thicknesses (see SEM cross-sectional images in Fig. 4(b)) were used as active channels and tested against 4.9 ppm of NO. The corresponding current profiles against long-time exposure (large dosage) are shown in Fig. 4. This film thickness-dependence is commonly observed in thin-film chemiresistor gas sensors based on the metal-oxide semiconductor,31,32 and carbon nanotube.33 The sensitivity-thickness correlation of gas sensors is usually based on the surface-to-volume ratio. However, in a real case, more complicated mechanisms involving surface-to-volume ratio, grain-size and boundary, edge effect,16 and porosity of the film32 have been used to explain the sensitivity-thickness correlation of thin-film chemiresistors. For our devices, the time required to reach 1/e of the conductance is 130, 180, and 230 min, that is, corresponding to a total dosage of 630, 880, and 1100 ppm min, for the thickness of 0.5 μm, 1.3 μm, and 11 μm, respectively. The detection range of 11 μm thin-film sensor reaches 1/20 of the dosage level of significant likelihood of death, given by experimental data from Health and Safety Executive, which makes it close to the practical application in the field test.
(a) The large dosage exposure of TaS2 devices with 3 different thicknesses. (b) The cross-sectional SEM images of the corresponding TaS2 films in (a).
(a) The large dosage exposure of TaS2 devices with 3 different thicknesses. (b) The cross-sectional SEM images of the corresponding TaS2 films in (a).
In summary, a room-temperature dosage meter of toxic gas is demonstrated using the TaS2 film consisting of the stacked TaS2 nanosheets exfoliated from the electrochemical lithium intercalation. The irreversible current response upon NO exposure enables the continuous record of the toxic load of NO gas in the sub-ppm level. Our study indicates the possibility to design electronic sensors based on the irreversible combination of conductive channel and target analtytes. In addition, the solution processability of two-dimensional nanosheets is promising for the low-cost, miniature, and portable solution for room-temperature gas sensors.
This work was supported by MOE under AcRF Tier 2 (ARC 26/13, No. MOE2013-T2-1-034), AcRF Tier 1 (RG 61/12, RGT18/13 and RG5/13), and Start-Up Grant (M4080865.070.706022) in Singapore. This Research is also conducted by NTU-HUJ-BGU Nanomaterials for Energy and Water Management Programme under the Campus for Research Excellence and Technological Enterprise (CREATE), that is supported by the National Research Foundation, Prime Minister's Office, Singapore.