Abstract
Although haplodiploid organisms tend to be inbred, previous models of the evolution of haplodiploidy have assumed outbred populations. Here a model for the evolution of haplodiploidy is developed which incorporates sib mating, deleterious mutations generated by mutation, and fitness differences between haploids and diploids. Simulations of the model allow an assessment of the effect of inbreeding on the deleterious mutation and maternal transmission theories for the evolution of haplodiploidy. As expected from intuitive arguments, inbreeding favours haplodiploidy under the deleterious mutation hypothesis but disfavours haplodiploidy under the maternal transmission hypothesis. It appears that the effect of inbreeding is greater on the maternal transmission theory, and thus inbreeding may restrict the evolution of haplodiploidy.
Similar content being viewed by others
Introduction
Sexual organisms are characterized by the ‘alternation of generations’ because the ploidy level is halved at meiosis and doubled at syngamy (Valero et al., 1992; Mable & Otto, 1998). Alternative sexual life cycles can be characterized by the timing of alternations between haploidy and diploidy. In diplonty somatic development takes place in the diploid phase, whereas in haplonty somatic development occurs in the haploid phase. In haplodiploid organisms the sexes differ in the ploidy level at which somatic development takes place: females develop as diploids whereas males develop as haploids. Diplonty is by far the most common life cycle found in animals, although there are two other common life cycles. One is asexual thelytoky, the evolution of which is addressed by the vast literature on the evolution of sex (see Hurst & Peck, 1996). The other is haplodiploidy, which appears to be a more stable and successful alternative to diplonty than asexuality (Borgia, 1980).
Although the evolutionary consequences of haplodiploidy have attracted a good deal of interest, especially in the context of social evolution (e.g. see Bourke & Franks, 1995), the evolutionary origins of haplodiploidy are still unclear (Whiting, 1945; Brown, 1964; Hartl & Brown, 1970; Bull, 1979; Borgia, 1980; Haig, 1993; Goldstein, 1994). The intention of this study is to discriminate between two hypotheses for the evolution of haplodiploidy from diplonty with respect to the effect of inbreeding. Although the number of independent origins of haplodiploidy is uncertain, it is generally agreed that haplodiploidy arose from diplonty (Hartl & Brown, 1970; Oliver, 1971; Borgia, 1980).
The maternal transmission hypothesis derives from the relatedness asymmetries of haplodiploidy. All the genes of a haplodiploid male come from his mother whereas only half of the genes of a diplontic male come from his mother. Thus a haplodiploidy modifier can spread in a diplontic population because of its enhanced transmission through the maternal germ line. Haplodiploidy can spread even if haploid males are less fit than diploid males, and thus the maternal transmission hypothesis invokes a selfish gene which spreads despite reducing population fitness. Haplodiploidy is predicted to evolve in outbred populations as long as haploid male fitness is more than half diploid male fitness. In this study I will develop a version of the maternal transmission hypothesis in which the modifier affects the transmission of the entire haploid genome, but it is possible that a similar process might act on a single chromosome. Indeed Hamilton (1993) has suggested that haplodiploidy might have evolved one chromosome at a time.
The deleterious mutation hypothesis suggests that a haplodiploidy modifier can spread in a diplontic population because of its indirect fitness consequences via selection on deleterious mutations (Goldstein, 1994). As with similar models for the transition between haplonty and diplonty (Otto & Goldstein, 1992), the spread of haplodiploidy is dependent on the strength of selection against deleterious mutations, the penetrance of deleterious mutations in diploids, and the level of recombination (Goldstein, 1994). Load arguments show that the mutational load in a haplodiploid population is lower than in a diplontic population (in outbred populations with no sex-ratio bias the haplodiploid load is 3/4 of the diplontic load). But the situation in an evolving population is more complex, as shown by modifier analysis. Purifying selection against deleterious mutations at viability loci is more efficient in haploids than in diploids if penetrance is incomplete, and so selection generates a linkage disequilibrium in which the haplodiploidy modifier associates with a higher fitness viability allele (and similarly the diplonty modifier associates with a lower fitness viability allele). In the absence of recombination, haplodiploidy is always favoured over diplonty. But if there is recombination then the linkage disequilibrium is reduced, which means that the cost of diplonty (association with a lower fitness viability allele) is reduced. If the rate of recombination is high enough, then diplonty can actually be favoured over haplodiploidy because of incomplete penetrance, because a diploid is less affected by a single deleterious mutation than is a haploid.
Inbreeding appears to be associated with haplodiploidy (Hamilton, 1967). Even if it is assumed that this association is the result of a causal relationship rather than the confounding effect of a third variable, and that the perceived association is robust to the demands of the comparative method, it is still not clear in which direction the causality lies. Haplodiploids may well complete the transition to inbreeding more easily than diplonts (Werren, 1993). Borgia (1980) has stressed the alternative view that inbreeding eases the transition from diplonty to haplodiploidy.
Intuition suggests that the reduction of mutation load relative to panmixia under inbreeding might be expected to favour haplodiploidy under the deleterious mutation hypothesis in two ways. First, inbreeding reduces the difference between diploid mutation load and haplodiploid mutation load: ‘diploid species appear to “benefit” more than haplodiploids from chronic inbreeding, in terms of a reduction in genetic load’ (Werren, 1993). This effect reduces the cost of the transition from diplonty to haplodiploidy, because the cost derives from the exposure of the standing body of deleterious recessives present in diplonts. The second reason is based on modifier analysis rather than load arguments. Inbreeding reduces the effectiveness of recombination. As argued above, a reduction in recombination ought to favour haplodiploidy over diplonty, because recombination acts to favour diplonty by breaking up the association between the haplodiploidy modifier and the higher fitness viability allele. The effect of inbreeding has already been investigated for the transition from diplonty to haplonty under a variety of mating schemes, and as predicted inbreeding favours haplonty over diplonty (Otto & Marks, 1996).
However, it also seems likely that inbreeding acts against the maternal transmission hypothesis for the evolution of haplodiploidy. Inbreeding reduces the proportion of heterozygotes, and the spread of the haplodiploidy modifier is favoured by transmission through females heterozygous at the ploidy modifier locus (Bull, 1979). The haplodiploidy modifier acts as a selfish gene and can only spread by virtue of its improved transmission relative to the diplonty modifier. In the extreme case, if inbreeding is complete, then the two ploidy modifier alleles can never meet, and so the haplodiploidy modifier can never spread.
The purpose of this study is to address two questions relating to the evolution of haplodiploidy under inbreeding. First, are the intuitive arguments correct in predicting the effect of inbreeding on the maternal transmission and deleterious mutation hypotheses? Secondly, can we predict the overall effect of inbreeding on the evolution of haplodiploidy?
The model
Goldstein’s (1994) model provides a scheme for incorporating both the maternal transmission and deleterious mutation hypotheses under panmixia. Using modifier analysis Goldstein was able to derive analytically the conditions under which a haplodiploidy modifier spreads in a diplont population at mutation–selection equilibrium. I have extended the model to include both random mating and sib mating. This change entails a considerable increase in the complexity of the algebra. Under panmixia, the recursions operate on a total of eight gamete frequencies, but under sib mating recursions have to be performed on the 90 possible adult mating-pairs. I have used a computer program to perform simulations by calculating the recursions automatically.
Consider two autosomal loci, one viability locus and one ploidy modifier locus. The two loci recombine at the rate r per meiosis. There are two alleles A and a at the viability locus. Allele A is wild-type with relative viability 1 in both homozygotes and hemizygotes (AA and A). Allele a is deleterious with relative viability 1 − s in homozygotes and hemizygotes (aa and a) and 1 − hs in heterozygotes (Aa). Thus the coefficient of selection against deleterious mutations is represented by s, and the penetrance of deleterious mutations in diploids is given by h. Mutation is unidirectional, from A to a at a rate μ per gamete.
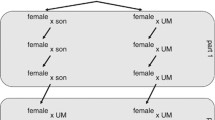
At the ploidy modifier locus there are two alleles P and p. As there are two alleles at both loci there is a total of four gamete types: Ap, ap, AP and aP. There are eight gamete frequencies which need to be specified in the model because gamete frequencies can differ between the sexes under haplodiploidy. Diploids produce gametes by meiosis, and haploid males are considered to produce sperm by a process analogous to mitosis. Diploid females are produced by the union of male and female gametes. The ploidy level of males depends on the genotype of their mother’s egg at the ploidy locus. If the female gamete contains P then the unfertilized egg develops into a haploid male, but if the female gamete contains p then the egg is fertilized and develops into a diploid male. In other words, P makes males out of unfertilized eggs. The control of sex determination is assumed to maintain a 1:1 sex ratio. The relative viability of haploid males vs. diploid males is given by haploid fitness f. The scheme under panmixia is shown in Fig. 1.
The breeding system is quantified by the variable sib, which represents the proportion of exclusively sib matings as opposed to random matings. The investigation of sib mating requires that recursions be performed on mating-pair frequencies rather than gamete frequencies. There are nine different male genotypes (ApAp, Apap, ApAP, ApaP, apap, apAP, apaP, AP, aP) and 10 different female genotypes (ApAp, Apap, ApAP, ApaP, apap, apAP, apaP, APAP, APaP, aPaP), so there is a total of 90 mating-pairs. For all mating-pairs the contributions of sib matings and random matings are combined in the proportions sib and 1 − sib, respectively.
Under the random mating scheme the proportions of the offspring types of each mating-pair are pooled across all mating-pairs weighted by mating-pair frequencies. It is assumed that each brood produces an effectively infinite number of offspring so that stochastic variation can be ignored. Gamete production and recombination, gamete mutation and random gamete fusion are invoked before the pooling of offspring types. Adults die after they have produced offspring, so do not contribute themselves to the next generation. Then viability selection followed by normalization acts on the pooled offspring. Finally, random mating-pairs are calculated by the products of the adult frequencies. This procedure carries the assumptions that mating is at random, that there is an effectively infinite number of adults, and that all adults mate.
Under the sib mating scheme new mating-pairs are generated within each mating-pair. Again an effectively infinite number of offspring within each brood ensures that all females are mated and that there is no stochastic variation. All the following processes therefore take place within the brood of each mating-pair: gamete production and recombination, gamete mutation, random gamete fusion to produce brood progeny, death of parents following reproduction, viability selection without normalization on offspring, and mating-pairs generated by the random union of surviving offspring within the brood. Following pooling across all mating-pairs, the new mating-pair frequencies generated by sib mating are normalized.
Invasion by haplodiploidy
For each simulation, values were assigned to the following parameters: μ, r, h, s, f and sib. The simulation began with the sole mating-pair as ApAp–ApAp and was allowed to proceed until the diplontic population reached mutation–selection equilibrium. Thus in the case of sib > 0 inbreeding was chronic, rather than acute (see Discussion). Then the P allele was introduced in the form of a small proportion (0.001) of the APAP–APAP mating-pair. The frequency of the P allele at the ploidy modifier locus was then calculated after 1000 and 2000 further generations, with the P allele frequencies at such times given by P1 and P2, respectively. If the P allele rose in frequency or had reached fixation (δP=P2 − P1 > 0 or P2=P1=1) then the haplodiploidy modifier had invaded the population. This simulation approach is similar to the methods of modifier analysis.
Panmixia
Under panmixia (sib=0) and no selection against deleterious mutations (s=0 or μ=0) the critical value of f is 1/2, as found in previous models of the maternal transmission hypothesis (Bull, 1979; Goldstein, 1994). Above this value haplodiploidy will always invade and below this value haplodiploidy can never invade.
If f is set to 1/2, then it is possible to examine the critical values for the invasion of haplodiploidy under the deleterious mutation hypothesis. The recombination rate r was set to 1/2 in all simulations under the assumption that a single ploidy modifier will be freely recombining with most loci in a genome with several chromosomes. The mutation rate μ was set to 0.0001 in all simulations. The critical values of h and s were in close agreement with Goldstein’s analytical results which were obtained by ignoring terms of the order of μ2 (see Fig. 2). High penetrance favours haplodiploidy because then the advantage to diplonty of the masking of deleterious mutations in diploids is reduced. Low recombination also favours haplodiploidy because mutation load effects which favour haplodiploidy over diploidy then increase in magnitude. The reduced recombination in haplodiploidy, caused by the lack of recombination in haploid males, explains why the conditions for the invasion of haplodiploidy are marginally less restrictive than the conditions for the invasion of full haplonty (Goldstein, 1994). More powerful selection against deleterious mutations also favours haplodiploidy (see Fig. 2) because stronger selection increases the association between the haplodiploidy modifier and the higher fitness viability allele.
The conditions for the invasion of diplonty by haplodiploidy in terms of selection against deleterious mutations (s) and penetrance (h) for different levels of sib mating (sib). The minimum value of h at which haplodiploidy can invade is given to a precision of 0.001. Goldstein refers to Goldstein’s (see eqn 5, 1994) analytically derived results for random mating (compare with sib=0).
Inbreeding
When sib mating is introduced into the model (1 ≥ sib > 0), interpretation of results becomes slightly more complicated because inbreeding affects both the maternal transmission and deleterious mutation arguments.
The effect of inbreeding on the maternal transmission hypothesis can be easily seen by removing selection against deleterious mutations (s=0 or μ=0). As expected, the critical value of f rises as the proportion of sib matings increases (see Fig. 3). When all matings are between sibs (sib=1) then haploid fitness must exceed diploid fitness ( f > 1) for haplodiploidy to invade.
It is not possible to consider the deleterious mutation hypothesis in isolation, but it is possible to account for the effects of maternal transmission. For a certain level of inbreeding, the critical value of f gives a minimal rate of haplodiploidy invasion inv (inv=δP). Although inv should clearly be zero at the critical point, unlimited precision cannot be achieved in simulations. However, the critical value of f was estimated by trial and error to a precision of 0.001, and so the resulting values of inv were very small. When selection against deleterious mutations is invoked, f is set to its critical value for the current value of sib and the requirement for haplodiploidy invasion then becomes δ P > inv rather than δP > 0. Thus it is possible to measure the effect of inbreeding on the deleterious mutation hypothesis. As expected, inbreeding facilitates the invasion of haplodiploidy according to the deleterious mutation hypothesis. For a given selective coefficient inbreeding reduces the minimum level of penetrance required for haplodiploidy to invade, once the effect of the maternal transmission hypothesis has been taken into account by adjusting f (see Table 1).
Discussion
The purpose of this study is to address two questions relating to the evolution of haplodiploidy under inbreeding. First, are intuitive arguments correct in predicting the effect of inbreeding on the maternal transmission and deleterious mutation hypotheses? Secondly, can we predict the overall effect of inbreeding on the evolution of haplodiploidy?
I have shown that intuitive arguments correctly predict the effect of inbreeding on the maternal transmission and deleterious mutation hypotheses for the evolution of haplodiploidy. Inbreeding favours the deleterious mutation argument and disfavours the maternal transmission argument. A more difficult question is whether such results tell us whether inbreeding makes the evolution of haplodiploidy more or less likely.
With a single viability locus the selective force of the maternal transmission hypothesis is far greater than the selective force of the deleterious mutation hypothesis. When a Pp female mates with a pp male, for example, half of the sons are P and half pp, whereas half of the daughters are Pp and half pp. Thus in terms of genetic composition of adults the frequency of P has jumped from 1/4 in the parents to 1/2 in the sons and 1/4 in the daughters. In contrast, P spreads much more slowly by the deleterious mutation hypothesis, at a rate of the order of the mutation rate.
This difference in rates of spread need not apply if multiple viability loci are invoked, as seems biologically realistic (Goldstein, 1994). If the modelling of multiple viability loci has the same effect on the transition from diplonty to haplodiploidy as on the transition from diplonty to haplonty, then the rate of spread of P becomes of the order of the per-genome mutation rate U (Jenkins & Kirkpatrick, 1995). Unless we know the size of U it is not possible to know which of the two hypotheses, maternal transmission or deleterious mutations, is likely to be the stronger (see below).
An alternative approach is to compare the sensitivity of the two hypotheses to increasing inbreeding. As can be seen from Table 1, inbreeding changes the critical value of f (maternal transmission theory) by considerably more than the critical value of h (deleterious mutation theory). Although we do not know the underlying probability distribution of either the f or h parameters (see below), this result does suggest that inbreeding may restrict the evolution of haplodiploidy, in contrast to previous conclusions (Borgia, 1980).
The conclusions of this study are dependent on the distributions of the parameters U, f and h. What do we know about these parameters?
The deleterious mutation rate per genome U has been estimated to be greater than unity in humans (Eyre-Walker & Keightley, 1999) and around unity in Drosophila (Simmons & Crow, 1977). These values suggest that the strength of the deleterious mutation effect may be just as strong as the strength of the maternal transmission effect.
Haploid males are likely to be weaker than diploid males for at least four reasons (Whiting, 1945; Borgia, 1980), so we can predict f < 1. First, males must develop from unfertilized eggs. Secondly, males may suffer from gene dosage problems. Thirdly, haploid males must be fertile, in other words spermatogenesis must work. Finally, haploid males will suffer from somatic deleterious mutations more than their diploid counterparts (Orr, 1995). From these considerations we might conclude that the maternal transmission hypothesis is unlikely to work if all matings are between sibs, because then the requirement for the invasion of haplodiploidy is f > 1. However, the considerations above need not apply under full inbreeding because haploid males would not enter into competition with diploid males and thus their weakness need not cause a corresponding decrease in fitness (Borgia, 1980).
Penetrance in Drosophila depends on the strength of selection (Simmons & Crow, 1977). Lethals are highly recessive (0.01 < h < 0.03) whereas mildly deleterious mutations show greater dominance (0.3 < h < 0.5). If genomic recombination is restricted (Goldstein, 1994) and/or if there is heavy inbreeding then the deleterious mutation hypothesis may favour haplodiploidy over diplonty.
This study has been carried out using a fairly simple model of haplodiploidy evolution. Several biological complexities have been ignored, the implications of which may well merit further study.
I have not considered the so-called parahaplodiploidy in which males develop from fertilized eggs but transmit only the female genome. The maternal transmission argument still applies to parahaplodiploidy, but the deleterious mutation theory seems unlikely to favour parahaplodiploidy over diplonty.
In the model studied here, I have assumed that the haplodiploidy modifier acts on the whole genome rather than on a single chromosome. However, the evolution of regional haplodiploidy on a single chromosome would probably be little different from the evolution of full haplodiploidy on the entire genome. Selection against deleterious mutations would be weaker for a single chromosome, which would reduce the deleterious mutation effect favouring haplodiploidy. On the other hand, recombination between loci on a single chromosome would be less than recombination between loci spread over the whole genome, which would favour haplodiploidy. There is also the possibility that chromosomes may differ in the penetrance and strength of selection against mutations. With regard to the maternal transmission hypothesis, the spread of a partial haplodiploidy would appear to be no different, in terms of population genetics, from the spread of full haplodiploidy. However, if only a subset of the genome is spreading selfishly, then perhaps there is greater scope for suppressor genes to evolve.
I have assumed that all mutations are deleterious, despite the fact that advantageous mutations must occur for adaptive evolution to proceed. Favourable mutations will appear at a higher rate in diploid males than in haploid males. If penetrance and recombination are both high then diplonty is favoured over haplonty (Orr & Otto, 1994), and the same conditions probably favour diplonty over haplodiploidy.
In some ways haplodiploidy might be thought of as a intermediate form between diplonty and haplonty, in which case it might not be clear why haplodiploidy is so evolutionarily stable. If the transition from diplonty to haplodiploidy is favoured, why not the transition from haplodiploidy to full haplonty? In the case of the deleterious mutation hypothesis, it is true that those conditions which favour haplodiploidy over diplonty will usually favour full haplonty over diplonty (Goldstein, 1994). This conclusion probably holds under inbreeding as well as outbreeding. In the case of the maternal transmission hypothesis it is hard to see how selfish genes could drive the evolution of full haplonty, although a selfish gene process could easily exploit the two-fold cost of sex to spread asexuality. The maintenance of haplodiploidy could then be explained by the instability of asexual lineages. Another possible answer to this problem is that gene dosage problems might be severe. Male haploidy may well be able to evolve despite the heavy costs of haploidy, but the costs of female haploidy may be too high if one considers the greater dependency of population fitness on female rather than male fitness.
Inbreeding raises a number of issues which have been avoided in this study. First, the model I have used does not specify sex determination, but is consistent with XX males and XO females which ensures that haploids are male. Alternative haplodiploid sex determination mechanisms may be costly under inbreeding (Werren, 1993). Secondly, haplodiploidy enables the primary sex ratio adjustment which is often favoured by inbreeding (although inbreeding is neither necessary nor sufficient for selection to favour biased sex ratios (Charnov, 1982)). However, this benefit can only be enjoyed after haplodiploidy has evolved and therefore cannot be considered as an explanation for the evolution of haplodiploidy. Thirdly, intragenomic conflict over the sex ratio might itself drive the evolution of haplodiploidy (Haig, 1993). This explanation is similar to the maternal transmission hypothesis in that intragenomic conflict is the driving force behind the evolution of genetic systems. Fourthly, another potential advantage of haplodiploidy associated with inbreeding is that haplodiploid mothers can produce sons with which to mate if population density is low (Hartl & Brown, 1970; Borgia, 1980).
One final complication not addressed here is the response to acute inbreeding. In this study I have assumed chronic inbreeding, so that the population reaches the mutation–selection equilibrium under inbreeding before the ploidy modifier is introduced. Alternatively, haplodiploidy may be favoured as a response to acute inbreeding. If inbreeding also favours a female bias to the sex ratio, then haplodiploidy would be especially favoured. Haplodiploidy would enable female fitness, and hence population fitness, to survive the transition from outbreeding to inbreeding relatively unscathed. Males, being haploid, would suffer from the exposure of deleterious mutations far more than the diploid females. But the population would not suffer as long as a few males were fertile. Under this scenario the evolution of sex-specific dispersal patterns would come about as a consequence of differences in mutation load. The weak males would be good for little more than inseminating their more vigorous sisters who would then go forth and multiply.
References
Borgia, G. (1980). Evolution of haplodiploidy: models for inbred and outbred systems. Theor Pop Biol, 17: 103–128.
Bourke, A. F. G. and Franks, N. R. (1995). Social Evolution in Ants, 1st edn. Princeton University Press, Princeton.
Brown, S. W. (1964). Automatic frequency response in the evolution of male haploidy and other coccid chromosomal systems. Genetics, 49: 797–817.
Bull, J. J. (1979). An advantage for the evolution of male haploidy and systems with similar genetic transmission. Heredity, 43: 361–381.
Charnov, E. L. (1982). The Theory of Sex Allocation, 1st edn. Princeton University Press, Princeton.
Eyre-Walker, A. and Keightley, P. D. (1999). High genomic deleterious mutation rates in hominids. Nature, 397: 344–347.
Goldstein, D. B. (1994). Deleterious mutations and the evolution of male haploidy. Am Nat, 144: 176–183.
Haig, D. (1993). The evolution of unusual chromosomal systems in coccoids: extraordinary sex ratios revisited. J Evol Biol, 6: 69–77.
Hamilton, W. (1993). Inbreeding in Egypt and in this book: a childish view. In: Thornhill, N. W. and Shields, W. M. (eds) The Natural History of Inbreeding and Outbreeding, pp. 429–450. University of Chicago Press, Chicago.
Hamilton, W. D. (1967). Extraordinary sex ratios. Science, 156: 477–488.
Hartl, D. L. and Brown, S. W. (1970). The origin of male haploid genetic systems and their expected sex ratio. Theor Pop Biol, 1: 165–190.
Hurst, L. D. and Peck, J. R. (1996). Recent advances in understanding of the evolution and maintenance of sex. Trends Ecol Evol, 11: 46–52.
Jenkins, C. D. and Kirkpatrick, M. (1995). Deleterious mutation and the evolution of genetic life cycles. Evolution, 49: 512–520.
Mable, B. K. and Otto, S. P. (1998). The evolution of life cycles with haploid and diploid phases. Bioessays, 20: 453–462.
Oliver, J. H. (1971). Parthenogenesis in mites and ticks (Arachnida: Acari). Am Zool, 11: 283–299.
Orr, H. A. (1995). Somatic mutation favors the evolution of diploidy. Genetics, 139: 1441–1447.
Orr, H. A. and Otto, S. P. (1994). Does diploidy increase the rate of adaptation? Genetics, 136: 1475–1480.
Otto, S. P. and Goldstein, D. B. (1992). Recombination and the evolution of diploidy. Genetics, 131: 745–751.
Otto, S. P. and Marks, J. C. (1996). Mating systems and the evolutionary transition between haploidy and diploidy. Biol J Linn Soc, 57: 197–218.
Simmons, M. J. and Crow, J. F. (1977). Mutations affecting fitness in Drosophila populations. Ann Rev Genet, 11: 49–78.
Valero, M., Richerd, S., Perrot, V. and Destombe, C. (1992). Evolution of alternation of haploid and diploid phases in life-cycles. Trends Ecol Evol, 7: 25–29.
Werren, J. H. (1993). The evolution of inbreeding in haplodiploid organisms. In: Thornhill, N. W. and Shields, W. M. (eds) The Natural History of Inbreeding and Outbreeding, pp. 42–59. University of Chicago Press, Chicago.
Whiting, P. W. (1945). The evolution of male haploidy. Q Rev Biol, 20: 231–260.
Acknowledgements
Many thanks to Laurence Hurst and two anonymous referees for many useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, N. The evolution of haplodiploidy under inbreeding. Heredity 84, 186–192 (2000). https://doi.org/10.1046/j.1365-2540.2000.00643.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00643.x
- Springer Nature Switzerland AG
Keywords
This article is cited by
-
Two sympatric lineages of Australian Cnestus solidus share Ambrosiella symbionts but not Wolbachia
Heredity (2023)
-
Genetic structure of Pseudococcus microcirculus (Hemiptera: Pseudococcidae) populations on epiphytic orchids in south Florida
Journal of Genetics (2017)
-
The Red Queen Process does not Select for High Recombination Rates in Haplodiploid Hosts
Evolutionary Biology (2013)