The distinct O2 quenching mechanism between fluorescence and phosphorescence for dyes adsorbed on silica gel†
Abstract
We herein aim to probe the emission quenched by O2 on silica gel. Our special focus is on the O2 quenching of the fluorescence of a series of organic D–π–A phosphonium compounds 1–3. The results show that the O2 quenching rate constants 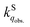 for the fluorescence of 1–3 are on the order of 1010 M−1 s−1, which are nearly on the same order as those measured for 1–3 and common organic compounds in solution. In yet another approach, the study of O2 quenching of phosphorescence in the solid phase indicates that the O2 quenching rate constant for the triplet state, i.e.,
for the fluorescence of 1–3 are on the order of 1010 M−1 s−1, which are nearly on the same order as those measured for 1–3 and common organic compounds in solution. In yet another approach, the study of O2 quenching of phosphorescence in the solid phase indicates that the O2 quenching rate constant for the triplet state, i.e., 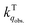 , is smaller than
, is smaller than 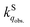 by two orders of magnitude. Detailed investigation indicates that this distinction stems from the intrinsic O2 quenching rate constants for the singlet and triplet states subsequent to the formation of collisional complexes. In the absence of the solvent cage effect,
by two orders of magnitude. Detailed investigation indicates that this distinction stems from the intrinsic O2 quenching rate constants for the singlet and triplet states subsequent to the formation of collisional complexes. In the absence of the solvent cage effect, 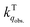 is greatly influenced by the formation energy of the O2–dye CT complex, whereas
is greatly influenced by the formation energy of the O2–dye CT complex, whereas 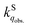 in the solid phase is a nearly diffusion-controlled rate. Due to the larger distinction between
in the solid phase is a nearly diffusion-controlled rate. Due to the larger distinction between 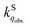 and
and 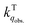 in the solid phase, O2 quenching of fluorescence is efficient for dyes in the solid phase. This leads to a feasible application of sensing O2 with regular fluorescent dyes adsorbed on porous solid substrates.
in the solid phase, O2 quenching of fluorescence is efficient for dyes in the solid phase. This leads to a feasible application of sensing O2 with regular fluorescent dyes adsorbed on porous solid substrates.



 Please wait while we load your content...
Please wait while we load your content...