Tracer diffusion of protons and oxide ions, as well as chemical diffusion of water, have been determined for the high temperature proton conductor lanthanum tungstate by means of time-of-flight secondary ion mass spectrometry (ToF-SIMS). The oxygen tracer diffusion and surface exchange coefficients, 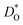 and
and 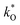 , were measured after exchange anneals in water vapor enriched in H218O between 350 and 620 °C, and the apparent activation energies were 176 and 82 kJ mol−1, respectively. The hydrogen tracer diffusion coefficient (
, were measured after exchange anneals in water vapor enriched in H218O between 350 and 620 °C, and the apparent activation energies were 176 and 82 kJ mol−1, respectively. The hydrogen tracer diffusion coefficient (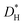 ) was measured between 220 and 320 °C after exchange anneals in D2O-containing atmospheres, and the apparent activation energy was 63 kJ mol−1. The extracted
) was measured between 220 and 320 °C after exchange anneals in D2O-containing atmospheres, and the apparent activation energy was 63 kJ mol−1. The extracted 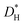 agrees with the results of transient conductivity- and TG-measurements. Chemical diffusion and surface exchange coefficients,
agrees with the results of transient conductivity- and TG-measurements. Chemical diffusion and surface exchange coefficients, 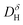 and
and 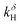 , were measured at 250 and 400 °C, and the result confirms that the material is hydrated by ambipolar sluggish transport of protons and oxide ions. The surface exchange coefficients were compared to the result of TG relaxation, suggesting that access to oxygen vacancies limits the overall surface exchange reaction under incorporation of water and oxygen.
, were measured at 250 and 400 °C, and the result confirms that the material is hydrated by ambipolar sluggish transport of protons and oxide ions. The surface exchange coefficients were compared to the result of TG relaxation, suggesting that access to oxygen vacancies limits the overall surface exchange reaction under incorporation of water and oxygen.

You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
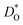 and
and 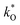 , were measured after exchange anneals in
, were measured after exchange anneals in 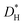 ) was measured between 220 and 320 °C after exchange anneals in D2O-containing atmospheres, and the apparent activation energy was 63 kJ mol−1. The extracted
) was measured between 220 and 320 °C after exchange anneals in D2O-containing atmospheres, and the apparent activation energy was 63 kJ mol−1. The extracted 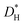 agrees with the results of transient conductivity- and TG-measurements. Chemical diffusion and surface exchange coefficients,
agrees with the results of transient conductivity- and TG-measurements. Chemical diffusion and surface exchange coefficients, 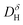 and
and 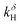 , were measured at 250 and 400 °C, and the result confirms that the material is hydrated by ambipolar sluggish transport of
, were measured at 250 and 400 °C, and the result confirms that the material is hydrated by ambipolar sluggish transport of 

 Please wait while we load your content...
Please wait while we load your content...