Freezing and melting behavior of an octyl β-d-glucoside–water binary system – inhibitory effect of octyl β-d-glucoside on ice crystal formation
Abstract
Phase transition behavior of lyotropic liquid crystals of an octyl β-D-glucoside (OG)–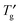 , were shown in the phase diagram. The phase diagram indicated that the OG aqueous system was concentrated to ca. 90–92 wt% by ice freezing and exhibited glass transition at
, were shown in the phase diagram. The phase diagram indicated that the OG aqueous system was concentrated to ca. 90–92 wt% by ice freezing and exhibited glass transition at 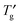 . An observation of the concentration-gradient specimen by the cryo-POM showed the evidence of the inhibitory effects of OG on nucleation and growth of ice crystals in the extremely high OG concentration system in which the lamellar liquid crystalline phase was formed. This study provided the importance of the influence of concentration change by ice freezing on the behaviour of the sugar-based
. An observation of the concentration-gradient specimen by the cryo-POM showed the evidence of the inhibitory effects of OG on nucleation and growth of ice crystals in the extremely high OG concentration system in which the lamellar liquid crystalline phase was formed. This study provided the importance of the influence of concentration change by ice freezing on the behaviour of the sugar-based


 Please wait while we load your content...
Please wait while we load your content...