Abstract
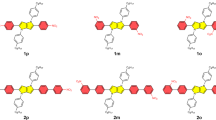
A bipyridine-based system with phenyleneethynylene at the 4,4’ positions (1) and its p-methyl (2) and p-methoxy (3) substituted derivatives were synthesized via Sonogashira coupling reactions. The photophysical properties of 1–3 and their related H+ and Zn2+ adducts (1:H+–3:H+ and 1:Zn2+–3:Zn2+) were investigated, as a function of solvent polarity, by using steady-state and time-resolved spectroscopic techniques. Molecular systems 1–3 exhibit trans conformation, whereas adducts with H+ and Zn2+ are conformationally locked cis species. The unsubstituted compound 1 emits at 360 nm with low fluorescence quantum yield (ϕfl = 0.2%) regardless of the solvent polarity. Fluorescence spectra of 2 and 3 are bathochromically shifted in polar solvents, and the p-methoxy (3) derivative possesses ϕfl as high as 12%. Complexation of 1–3 with H+ or Zn2+ in acetonitrile causes red-shift of the lowest energy absorption bands, whereas dramatic changes of the emission properties are found as a function of the electron donating ability of the substituents on the phenyleneethynylene moiety (–CH3 or–OCH3), suggesting a charge-transfer character of the lowest electronic transition of 1–3. 1:H+, 1:Zn2+, 2:H+ and 2:Zn2+ exhibit intense fluorescence with ϕfl up to 33% (1:Zn2+) whilst 3:H+ and 3:Zn2+ are found to be weakly emissive. The singlet radiative and non-radiative rate constants of compounds and complexes were determined, along with triplet parameters, via phosphorescence and transient absorption spectroscopy. More conclusive evidence regarding the protonation of bipyridine nitrogen atoms of compounds 1–3 were obtained through 1H NMR titration studies. These studies indicate that the conjugate molecular systems based on 2,2’-bipyridine and phenyleneethenylenes possess tunable optical properties which can be further utilized for preparing organic and inorganic luminophores with potential application in optoelectronic systems
Similar content being viewed by others
Notes and references
A. P. de Silva, T. P. Vance, M. E. S. West and G. D. Wright, Bright molecules with sense, logic, numeracy and utility, Org. Biomol. Chem., 2008, 6, 2468–2480.
Y. R. Sun, N. C. Giebink, H. Kanno, B. W. Ma, M. E. Thompson and S. R. Forrest, Management of singlet and triplet excitons for efficient white organic light-emitting devices, Nature, 2006, 440, 908–912.
J. L. Bredas, D. Beljonne, V. Coropceanu and J. Cornil, Charge-transfer and energy-transfer processes in pi-conjugated oligomers and polymers: A molecular picture, Chem. Rev., 2004, 104, 4971–5003.
D. Katsis, Y. H. Geng, J. J. Ou, S. W. Culligan, A. Trajkovska, S. H. Chen and L. J. Rothberg, Spiro-linked ter-, penta-, and heptafluorenes as novel amorphous materials for blue light emission, Chem. Mater., 2002, 14, 1332–1339.
J. Kido and Y. Okamoto, Organo lanthanide metal complexes for electroluminescent materials, Chem. Rev., 2002, 102, 2357–2368.
Y. Eichen, G. Nakhmanovich, V. Gorelik, O. Epshtein, J. M. Poplawski and E. Ehrenfreund, Effect of protonation-deprotonation processes on the electrooptical properties of bipyridine-containing poly(p-phenylene-vinylene) derivatives, J. Am. Chem. Soc., 1998, 120, 10463–10470.
S. Leroy-Lhez and F. Fages, Polypyridine ligands with extended pi-conjugation: highly tunable fluorophores, C. R. Chim., 2005, 8, 1204–1212.
C. O. Dietrich-Buchecker, J. P. Sauvage, N. Armaroli, P. Ceroni and V. Balzani, Protonation-driven formation of a double-stranded structure: a photophysical and 1H-NMR study, New J. Chem., 1996, 20, 801–808.
N. Armaroli, P. Ceroni, V. Balzani, J. M. Kern, J. P. Sauvage and J. L. Weidmann, Protonation of free 2,9-bis(p-biphenylyl)-1,10-phenanthroline sites in a 56-membered macrocycle and in its ReI and CuI complexes. Absorption spectra, luminescence properties, and excited state interactions, J. Chem. Soc., Faraday Trans., 1997, 93, 4145–4150.
H. S. Joshi, R. Jamshidi and Y. Tor, Conjugated 1,10-phenanthrolines as tunable fluorophores, Angew. Chem., Int. Ed., 1999, 38, 2721–2725.
A. Listorti, A. D. Esposti, R. S. K. Kishore, V. Kalsani, M. Schmittel and N. Armaroli, 1,10-phenanthrolines with tunable luminescence upon protonation: A spectroscopic and computational study, J. Phys. Chem. A, 2007, 111, 7707–7718.
T. Renouard, H. Le Bozec, S. Brasselet, I. Ledoux and J. Zyss, Tetrahedral bipyridyl copper(I) complexes: a new class of non-dipolar chromophore for nonlinear optics, Chem. Commun., 1999, 871–872.
J. C. Loren and J. S. Siegel, Synthesis and fluorescence properties of manisyl-substituted terpyridine, bipyridine, and phenanthroline, Angew. Chem., Int. Ed., 2001, 40, 754–757.
Y. Liu, Y. Li and K. S. Schanze, Photophysics of p-conjugated oligomers and polymers that contain transition metal complexes, J. Photochem. Photobiol., C, 2002, 3, 1–23.
N. Armaroli, From metal complexes to fullerene arrays: exploring the exciting world of supramolecular photochemistry fifteen years after its birth, Photochem. Photobiol. Sci., 2003, 2, 73–87.
L. Viau, S. Bidault, O. Maury, S. Brasselet, I. Ledoux, J. Zyss, E. Ishow, K. Nakatani, H. Le Bozec, All-optical orientation of photoisomerizable octupolar zinc(II) complexes in polymer films, J. Am. Chem. Soc., 2004, 126, 8386–8387.
M. Schmittel, V. Kalsani, C. Michel, P. Mal, H. Ammon, F. Jackel and J. P. Rabe, Towards nanotubular structures with large voids: Dynamic heteroleptic oligophenanthroline metallonanoscaffolds and their solution-state properties, Chem.–Eur.J., 2007, 13, 6223–6237.
M. W. Cooke, D. Chartrand and G. S. Hanan, Self-assembly of discrete metallosupramolecular luminophores, Coord. Chem. Rev., 2008, 252, 903–921.
G. Accorsi, A. Listorti, K. Yoosaf and N. Armaroli, 1,10-Phenanthrolines: versatile building blocks for luminescent molecules, materials and metal complexes, Chem. Soc. Rev., 2009, 38, 1690–1700.
C. A. Breen, J. R. Tischler, V. Bulovic and T. M. Swager, Highly efficient blue electroluminescence from poly(phenylene ethynylene) via energy transfer from a hole-transport matrix, Adv. Mater., 2005, 17, 1981–1985.
D. K. James and J. M. Tour, Molecular wires: From design to properties, Top. Curr. Chem., 2005, 257, 33–62.
P. V. James, P. K. Sudeep, C. H. Suresh and K. G. Thomas, Photophysical and theoretical investigations of oligo(p-phenyleneethynylene)s: Effect of alkoxy substitution and alkyne-aryl bond rotations, J. Phys. Chem. A, 2006, 110, 4329–4337.
I. B. Kim, R. Phillips, U. H. F. Bunz, Forced agglutination as a tool to improve the sensory response of a carboxylated poly(p-phenyleneethynylene), Macromolecules, 2007, 40, 814–817.
H. Li, D. R. Powell, R. K. Hayashi and R. West, Poly((2,5-dialkoxy-p-phenylene)ethynylene-p-phenyleneethynylene)s and their model compounds, Macromolecules, 1998, 31, 52–58.
H. Li and R. West, Structures and photophysical properties of silicon-containing phenyleneethynylene polymers, Macromolecules, 1998, 31, 2866–2871.
J. F. Nierengarten, S. Zhang, A. Gegout, M. Urbani, N. Armaroli, G. Marconi and Y. Rio, Synthesis and optical properties of isomeric branched pi-conjugated systems, J. Org. Chem., 2005, 70, 7550–7557.
Y. Shirai, J. F. Morin, T. Sasaki, J. M. Guerrero and J. M. Tour, Recent progress on nanovehicles, Chem. Soc. Rev., 2006, 35, 1043–1055.
P. K. Sudeep, P. V. James, K. G. Thomas and P. V. Kamat, Singlet and triplet excited-state interactions and photochemical reactivity of phenyleneethynylene oligomers, J. Phys. Chem. A, 2006, 110, 5642–5649.
W. Zhang and J. S. Moore, Shape-persistent macrocycles: Structures and synthetic approaches from arylene and ethynylene building blocks, Angew. Chem., Int. Ed., 2006, 45, 4416–4439.
X. Y. Zhao, M. R. Pinto, L. M. Hardison, J. Mwaura, J. Muller, H. Jiang, D. Witker, V. D. Kleiman, J. R. Reynolds and K. S. Schanze, Variable band gap poly(arylene ethynylene) conjugated polyelectrolytes, Macromolecules, 2006, 39, 6355–6366.
L. T. Liu, D. Yaron and M. A. Berg, Electron-phonon coupling in phenyleneethynylene oligomers: A nonlinear one-dimensional configuration-coordinate model, J. Phys. Chem. C, 2007, 111, 5770–5782.
M. Levitus, K. Schmieder, H. Ricks, K. D. Shimizu, U. H. F. Bunz and M. A. Garcia-Garibay, Steps to demarcate the effects of chromophore aggregation and planarization in poly(phenyleneethynylene)s. 1. Rotationally interrupted conjugation in the excited states of 1,4-bis(phenylethynyl)benzene, J. Am. Chem. Soc., 2001, 123, 4259–4265; addition/correction
M. Levitus, K. Schmieder, H. Ricks, K. D. Shimizu, U. H. F. Bunz, M. A. Garcia-Garibay, J. Am. Chem. Soc., 2002, 124, 8181.
A. Beeby, K. Findlay, P. J. Low and T. B. Marder, A re-evaluation of the photophysical properties of 1,4-bis(phenylethynyl)benzene: A model for poly(phenyleneethynylene), J. Am. Chem. Soc., 2002, 124, 8280–8284.
L. Zhao, I. F. Perepichka, F. Turksoy, A. S. Batsanov, A. Beeby, K. S. Findlay and M. R. Bryce, 2,5-di(aryleneethynyl)pyrazine derivatives: synthesis, structural and optoelectronic properties, and light-emitting device, New J. Chem., 2004, 28, 912–918.
T. Terashima, T. Nakashima and T. Kawai, Engineering control over the conformation of the alkyne-aryl bond by the introduction of cationic charge, Org. Lett., 2007, 9, 4195–4198.
A. Beeby, K. S. Findlay, P. J. Low, T. B. Marder, P. Matousek, A. W. Parker, S. R. Rutter and M. Towrie, Studies of the S1, state in a prototypical molecular wire using picosecond time-resolved spectroscopies, Chem. Commun., 2003, 2406–2407.
Y. Matsunaga, K. Takechi, T. Akasaka, A. R. Ramesh, P. V. James, K. G. Thomas and P. V. Kamat, Excited-State and photoelectrochemical behavior of pyrene-linked phenyleneethynylene oligomer, J. Phys. Chem. B, 2008, 112, 14539–14547.
A. Llanes-Pallas, C.-A. Palma, L. Piot, A. Belbakra, A. Listorti, M. Prato, P. Samorì, N. Armaroli and D. Bonifazi, Engineering of supramolecular H-bonded nanopolygons via self-assembly of programmed molecular modules, J. Am. Chem. Soc., 2009, 131, 509–520.
Y. Yamaguchi, T. Ochi, T. Wakamiya, Y. Matsubara and Z. Yoshida, New fluorophores with rod-shaped polycyano pi-conjugated structures: Synthesis and photophysical properties, Org. Lett., 2006, 8, 717–720.
Y. L. Tang, Z. J. Zhou, K. Ogawa, G. P. Lopez, K. S. Schanze and D. G. Whitten, Synthesis, self-assembly, and photophysical behavior of oligo phenylene ethynylenes: from molecular to supramolecular properties, Langmuir, 2009, 25, 21–25.
A. De Nicola, Y. Liu, K. S. Schanze and R. Ziessel, One-pot synthesis of 2,5-diethynyl-3,4-dibutylthiophene substituted multitopic bipyridine ligands: redox and photophysical properties of their ruthenium(II) complexes, Chem. Commun., 2003, 288–289.
K. D. Glusac, S. J. Jiang and K. S. Schanze, Photophysics of Ir(III) complexes with oligo(arylene ethynylene) ligands, Chem. Commun., 2002, 2504–2505.
U. W. Grummt, E. Birckner, E. Klemm, D. A. M. Egbe and B. Heise, Conjugated polymers with 2,2’ -bipyridine and diethinylenebenzene units: absorption and luminescence properties, J. Phys. Org. Chem., 2000, 13, 112–126.
E. Birckner, U. W. Grummt, A. H. Goller, T. Pautzsch, D. A. M. Egbe, M. Al-Higari and E. Klemm, Photophysics of arylene and heteroaryleneethinylenes, J. Phys. Chem. A, 2001, 105, 10307–10315.
S. Leroy-Lhez, A. Parker, P. Lapouyade, C. Belin, L. Ducasse, J. Oberle and F. Fages, Tunable fluorescence emission in pyrene-(2,2’ -bipyridine) dyads containing phenylene-ethynylene bridges, Photochem. Photobiol. Sci., 2004, 3, 949–958.
N. Armaroli, L. De Cola, V. Balzani, J. P. Sauvage, C. O. Dietrich-Buchecker and J. M. Kern, Absorption and luminescence properties of 1, 10-phenanthroline, 2,9-diphenyl-1, 10-phenanthroline, 2,9-dianisyl-1,10- phenanthroline and their protonated forms in dichloromethane solution, J. Chem. Soc., Faraday Trans., 1992, 88, 553–556.
C. L. Cheng, D. S. N. Murthy, G. L. D. Ritchie, Molecular conformations from magnetic anisotropies, J. Chem. Soc., Faraday Trans. 2, 1972, 68, 1679–1690.
S. T. Howard, Conformers, energetics, and basicity of 2,2’-bipyridine, J. Am. Chem. Soc., 1996, 118, 10269–10274.
F. H. Westheimer and O. T. Benfey, The quantitative evaluation of the effect of hydrogen bonding on the strength of dibasic acids, J. Am. Chem. Soc., 1956, 78, 5309–5311.
D. E. C. Corbridge and E. G. Cox, Five-covalent terpyridyl complexes of bivalent metals. Part I. The, stereochemistry of the zinc, cadmium, and copper compounds, J. Chem. Soc., 1956, 594–603.
K. Nakamoto, Ultraviolet spectra and structures of 2,2’-bipyridine and 2,2’,2”-terpyridine in aqueous solution, J. Phys. Chem., 1960, 64, 1420–1425.
A. E. Dennis and R. C. Smith, “Turn-on” fluorescent sensor for the selective detection of zinc ion by a sterically-encumbered bipyridyl-based receptor, Chem. Commun., 2007, 4641–4643.
A. Ajayaghosh, P. Carol and S. Sreejith, A ratiometric fluorescence probe for selective visual sensing of Zn2+, J. Am. Chem. Soc., 2005, 127, 14962–14963.
N. Armaroli, L. De Cola, V. Balzani, J. P. Sauvage, C. O. Dietrich-Buchecker, J. M. Kern and A. Bailal, Absorption and emission properties of a 2-catenand, its protonated forms, and its complexes with Li+, Cu+, Ag+, Co2+, Ni2+, Zn2+, Pd2+ and Cd2+ - Tuning of the luminescence over the whole visible spectral region, J. Chem. Soc., Dalton Trans., 1993, 3241–3247.
J. N. Demas and G. A. Crosby, Measurement of photoluminescence quantum yields - Review, J. Phys. Chem., 1971, 75, 991–1024.
S. R. Meech and D. Phillips, Photophysics of some common fluorescence standards, J. Photochem., 1983, 23, 193–217.
K. Nakamaru, Synthesis, luminescence quantum yields, and lifetimes of Tris chelated Ru(II) mixed-ligand complexes including 3,3’-dimethyl-2,2’-bipyridyl, Bull. Chem. Soc. Jpn., 1982, 55, 2697–2705.
K. Hosomizu, H. Imahori, U. Hahn, J. F. Nierengarten, A. Listorti, N. Armaroli, T. Nemoto and S. Isoda, Dendritic effects on structure and photophysical and photoelectrochemical properties of fullerene dendrimers and their nanoclusters, J. Phys. Chem. C, 2007, 111, 2777–2786.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
James, P.V., Yoosaf, K., Kumar, J. et al. Tunable photophysical properties of phenyleneethynylene based bipyridine ligands. Photochem Photobiol Sci 8, 1432–1440 (2009). https://doi.org/10.1039/b9pp00002j
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b9pp00002j