Exothermic laws applicable to the degradation of o-phenylenediamine in wastewater via a Fe3+/H2O2 homogeneous quasi-Fenton system
Abstract
We studied the exothermic laws of Fe3+/H2O2 homogeneous quasi-Fenton degradation of o-phenylenediamine in waste water, and analyzed the effects of [H2O2] and [Fe3+], initial reaction temperature, and other factors on the solution temperature elevation (Δt), temperature elevation duration (T), and chemical oxygen demand degradation rate (η) during the degradation of the target pollutant. Our study found that [H2O2] is a major factor affecting Δt, while [Fe3+] and t0 are the main factors influencing the exothermic reaction rate. For the conditions wherein [H2O2] is 0.2 mol L−1, [Fe3+] is 10 mmol L−1, pH = 7.8, initial reaction temperature is 30 °C, and reaction duration is 30 min, Δt of 200 mL of 0.04 mol L−1 o-phenylenediamine is 7.2 °C and η is 93.45%. The exothermic reaction between the free radicals (·OH and 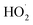 ) and o-phenylenediamine and the exothermic reaction due to auto-consumption of free radicals are the main reasons for the increased temperature of the solution.
) and o-phenylenediamine and the exothermic reaction due to auto-consumption of free radicals are the main reasons for the increased temperature of the solution.



 Please wait while we load your content...
Please wait while we load your content...