Abstract
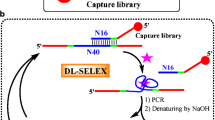
Aptamers are short single-stranded DNA or RNA, which can be selected in vitro by systematic evolution of ligands by exponential enrichment (SELEX). In order to develop novel light-up probes to substitute G-quadruplex (G4), we selected a DNA aptamer for crystal violet (CV), a triphenylmethane light-up dye, by a modified affinity chromatography-based SELEX. The ssDNA pool was first coupled on streptavidin-coated agarose beads through a biotin labeled complementary oligonucleotide, and then the aptamer sequences would be released from agarose beads by CV affinity. This method is simple, straightforward and effective. The aptamer sequence with a low micromolar dissociation constant (Kd) and good specificity was achieved after 11 rounds of selection. The light-up properties of the CV–aptamer were also investigated, and the CV showed dramatic fluorescence enhancement. The CV–aptamer pair could be further used as a novel light-up fluorescent probe to design biosensors.
Similar content being viewed by others
References
J. T. Davis, G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry, Angew. Chem., Int. Ed., 2004, 43, 668–698.
S. Millevoi, H. Moine and S. Vagner, G-quadruplexes in RNA biology, WIREs RNA, 2012, 3, 495–507.
A. T. Phan, Human telomeric G-quadruplex: structures of DNA and RNA sequences, FEBS J., 2010, 277, 1107–1117.
Y. Xu, Chemistry in human telomere biology: structure, function and targeting of telomere DNA/RNA, Chem. Soc. Rev., 2011, 40, 2719–2740.
A. M. Burger, F. Dai, C. M. Schultes, A. P. Reszka, M. J. Moore, J. A. Double and S. Neidle, The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function, Cancer Res., 2005, 65, 1489–1496.
S. Burge, G. N. Parkinson, P. Hazel, A. K. Todd and S. Neidle, Quadruplex DNA: sequence, topology and structure, Nucleic Acids Res., 2006, 34, 5402–5415.
B. Li, S. Dong and E. Wang, Homogeneous analysis: label-free and substrate-free aptasensors, Chem. - Asian J., 2010, 5, 1262–1272.
E. Largy, A. Granzhan, F. Hamon, D. Verga and M. P. Teulade-Fichou, Visualizing the quadruplex: from fluorescent Iigands to light-up probes, Top. Curr. Chem., 2013, 330, 111–177.
B. R. Vummidi, J. Alzeer and N. W. Luedtke, Fluorescent probes for G-quadruplex structures, ChemBioChem, 2013, 14, 540–558.
H. Arthanari, S. Basu, T. L. Kawano and P. H. Bolton, Fluorescent dyes specific for quadruplex DNA, Nucleic Acids Res., 1998, 26, 3724–3728.
W. J. Zhang, T. M. Ou, Y. J. Lu, Y. Y. Huang, W. B. Wu, Z. S. Huang, J. L. Zhou, K. Y. Wong and L. Q. Gu, 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA, Bioorg. Med. Chem., 2007, 15, 5493–5501.
A. C. Bhasikuttan, J. Mohanty and H. Pal, Interaction of malachite green with guanine-rich single-stranded DNA: preferential binding to a G-quadruplex, Angew. Chem., Int. Ed., 2007, 46, 9305–9307.
Z. Zhang, E. Sharon, R. Freeman, X. Liu and I. Willner, Fluorescence detection of DNA, adenosine-5′-triphosphate (ATP), and telomerase activity by zinc(II)-protoporphyrin IX/G-quadruplex labels, Anal. Chem., 2012, 84, 4789–4797.
D. Hu, F. Pu, Z. Huang, J. Ren and X. Qu, A quadruplex-based, label-free, and real-time fluorescence assay for RNase H activity and inhibition, Chemistry, 2010, 16, 2605–2610.
H. Z. He, V. P. Ma, K. H. Leung, D. S. Chan, H. Yang, Z. Cheng, C. H. Leung and D. L. Ma, A label-free G-quadruplex-based switch-on fluorescence assay for the selective detection of ATP, Analyst, 2012, 137, 1538–1540.
J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA, J. Am. Chem. Soc., 2013, 135, 367–376.
D. Shangguan, Y. Li, Z. Tang, Z. C. Cao, H. W. Chen, P. Mallikaratchy, K. Sefah, C. J. Yang and W. Tan, Aptamers evolved from live cells as effective molecular probes for cancer study, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11838–11843.
Y. Guo, W. Yao, Y. Xie, X. Zhou, J. Hu and R. Pei, Logic gates based on G-quadruplexes: principles and sensor applications, Microchim. Acta, 2016, 183, 21–34.
C. Tuerk and L. Gold, Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase, Science, 1990, 249, 505–510.
A. D. Ellington and J. W. Szostak, In vitro selection of RNA molecules that bind specific ligands, Nature, 1990, 346, 818–822.
Y. Yang, D. Yang, H. J. Schluesener and Z. Zhang, Advances in SELEX and application of aptamers in the central nervous system, Biomol. Eng., 2007, 24, 583–592.
M. Famulok, G. Mayer and M. Blind, Nucleic acid aptamers-from selection in vitro to applications in vivo, Acc. Chem. Res., 2000, 33, 591–599.
Y. Du, B. Li and E. Wang, “Fitting” makes “sensing” simple: label-free detection strategies based on nucleic acid aptamers, Acc. Chem. Res., 2013, 46, 203–213.
H. Wang, H. Cheng, J. Wang, L. Xu, H. Chen and R. Pei, Selection and characterization of DNA aptamers for the development of light-up biosensor to detect Cd(II), Talanta, 2016, 154, 498–503.
D. M. Kolpashchikov, Binary malachite green aptamer for fluorescent detection of nucleic acids, J. Am. Chem. Soc., 2005, 127, 12442–12443.
C. H. Leung, D. S. Chan, B. Y. Man, C. J. Wang, W. Lam, Y. C. Cheng, W. F. Fong, W. L. Hsiao and D. L. Ma, Simple and convenient G-quadruplex-based turn-on fluorescence assay for 3′ >5′ exonuclease activity, Anal. Chem., 2011, 83, 463–466.
J. R. Babendure, S. R. Adams and R. Y. Tsien, Aptamers switch on fluorescence of triphenylmethane dyes, J. Am. Chem. Soc., 2003, 125, 14716–14717.
S. Sando, A. Narita and Y. Aoyama, Light-up Hoechst-DNA aptamer pair: generation of an aptamer-selective fluorophore from a conventional DNA-staining dye, ChemBioChem, 2007, 8, 1795–1803.
T. P. Constantin, G. L. Silva, K. L. Robertson, T. P. Hamilton, K. Fague, A. S. Waggoner and B. A. Armitage, Synthesis of new fluorogenic cyanine dyes and incorporation into RNA fluoromodules, Org. Lett., 2008, 10, 1561–1564.
H. Arthanari, S. Basu, T. L. Kawano and P. H. Bolton, Fluorescent dyes specific for quadruplex DNA, Nucleic Acids Res., 1998, 26, 3724–3728.
D. M. Kong, Y. E. Ma, J. Wu and H. X. Shen, Discrimination of G-Quadruplexes from duplex and single-stranded DNAs with fluorescence and energy-transfer fluorescence spectra of crystal violet, Chem. - Eur. J., 2009, 15, 901–909.
F. Li, Y. Feng, C. Zhao and B. Tang, Crystal violet as a G-quadruplex-selective probe for sensitive amperometric sensing of lead, Chem. Commun., 2011, 47, 11909–11911.
Y. Jin, J. Bai and H. Li, Label-free protein recognition using aptamer-based fluorescence assay, Analyst, 2010, 135, 1731–1735.
M. Rajendran and A. D. Ellington, In vitro selection of molecular beacons, Nucleic Acids Res., 2003, 31, 5700–5713.
R. Nutiu and Y. Li, In vitro selection of structure-switching signaling aptamers, Angew. Chem., Int. Ed., 2005, 44, 1061–1065.
T. L. Bailey and C. Elkan, Fitting a mixture model by expectation maximization to discover motifs in biopolymers, Proc. - Int. Conf. Intell. Syst. Mol. Biol., 1994, 2,28-36.
R. Beinoraviciute-Kellner, G. Lipps and G. Krauss, In vitro selection of DNA binding sites for ABF1 protein from Saccharomyces cerevisiae, FEBS Lett., 2005, 579, 4535–4540.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21575154, 21775160, 21507156) and the Science and Technology Foundation of Suzhou (No. SYG201526).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, J., Zhang, Y. et al. Selection and characterization of a DNA aptamer to crystal violet. Photochem Photobiol Sci 17, 800–806 (2018). https://doi.org/10.1039/c7pp00457e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00457e