Abstract
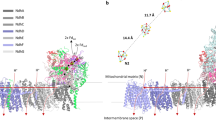
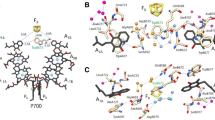
With available high resolution structures of PSII and a collection of reported redox midpoint potentials for most of the cofactors, it is possible to compare the expected electron tunneling rates with experimental rates to determine which electron transfer reactions are likely to reflect simply engineered electron tunneling, and which are more sophisticated and associated with large product rearrangements or the making and breaking of bonds. Reliable reorganization energies are largely lacking in this photosystem compared to PSI and purple bacteria and contribute about an order of magnitude uncertainty in tunneling rate estimates. Nevertheless it seems clear that as in purple bacterial reaction centers and PSI, with the notable exception of the oxygen evolving center, the majority of electron transfers within PSII are electron-tunneling limited at room temperature. Tunneling simulations also suggest that the short circuit between pheophytin and the adjacent chlorophyll cation may be fast enough to challenge triplet decay as the principle means of charge recombination from QA− at room temperature.
Similar content being viewed by others
References
C. C. Moser, J. M. Keske, K. Warncke, R. S. Farid and P. L. Dutton, Nature of biological electron transfer Nature (London) 1992 355 796–802
C. C. Page, C. C. Moser, X. Chen and P. L. Dutton, Natural engineering principles of electron tunneling in biological oxidation-reduction Nature (London) 1999 402 47–52
K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Architecture of the photosynthetic oxygen-evolving center Science 2004 303 1831–1838
A. Krieger, A. W. Rutherford and G. N. Johnson, On the Determination of Redox Midpoint Potential of the Primary Quinone Electron-Acceptor, Q(a), in Photosystem-II Biochim. Biophys. Acta 1995 1229 193–201
A. W. Rutherford, J. E. Mullet and A. R. Crofts, Measurement of the Midpoint Potential of the Pheophytin Acceptor of Photosystem-II FEBS Lett. 1981 123 235–237
F. Rappaport, M. Guergova-Kuras, P. J. Nixon, B. A. Diner and J. Lavergne, Kinetics and pathways of charge recombination in photosystem II Biochemistry 2002 41 8518–8527
G. N. Johnson, A. W. Rutherford and A. Krieger, A Change in the Midpoint Potential of the Quinone Q(a) in Photosystem-II Associated with Photoactivation of Oxygen Evolution Biochim. Biophys. Acta 1995 1229 202–207
B. G. Degrooth and H. J. Vangorkom, External Electric-Field Effects on Prompt and Delayed Fluorescence in Chloroplasts Biochim. Biophys. Acta 1981 635 445–456
B. A. Diner et al. Site-directed mutations at D1-His198 and D2-His 97 of photosystem II in synechocystis PCC 6803: Sites of primary charge separation and cation and triplet stabilization Biochemistry 2001 40 9265–9281
H. Ishikita and E. W. Knapp, Redox Potentials of chlorophylls and beta-carotene in the antenna complexes of photosystem II J. Am. Chem. Soc. 2005 127 1963–1968
R. A. Marcus, On the theory of oxidation-reduction reactions involving electron transfer: I. J. Chem. Phys. 1956 24 966–978
R. A. Marcus and N. Sutin, Electron transfers in chemistry and biology Biochim. Biophys. Acta 1985 811 265–322
C. C. Moser and P. L. Dutton, Engineering protein structure for electron transfer function in photosynthetic reaction centers Biochim. Biophys. Acta 1992 1101 171–176
C. C. Moser, C. C. Page, X. Chen and P. L. Dutton, in Enzyme-Catalyzed Electron Radical Transfer, ed. N. S. Scrutton, Plenum/Kluwer Press, The Netherlands, 2000, pp. 1-30
M. R. Gunner and P. L. Dutton, Temperature and ΔG-Degrees Dependence of the Electron-Transfer from Bph.- to Qa in Reaction Center Protein from Rhodobacter-Sphaeroides with Different Quinones As Qa J. Am. Chem. Soc. 1989 111 3400–3412
G. Renger, Mechanistic and structural aspects of photosynthetic water oxidation Physiol. Plant. 1997 100 828–841
G. Renger, G. Christen, M. Karge, H. J. Eckert and K. D. Irrgang, Application of the Marcus theory for analysis of the temperature dependence of the reactions leading to photosynthetic water oxidation: results and implications J. Biol. Inorg. Chem. 1998 3 360–366
C. Jeans, M. J. Schilstra and D. R. Klug, The temperature dependence of P680+ reduction in oxygen-evolving photosystem II Biochemistry 2002 41 5015–5023
J. J. Hopfield, Electron transfer between biological molecules by thermally activated tunneling Proc. Natl. Acad. Sci. USA 1974 71 3640–3644
V. G. Levich and R. R. Dogonadze, Teiriya bezizluchatelnikh electronnikh perekhodov mezhdu ionami v rastvorakh Dokl. Akad. Nauk. SSSR. 1959 124 123–6
A. Cuni, L. Xiong, R. Sayre, F. Rappaport and J. Lavergne, Modification of the pheophytin midpoint potential in photosystem II: Modulation of the quantum yield of charge separation and of charge recombination pathways Phys. Chem. Chem. Phys. 2004 6 4825–4831
K. K. Karukstis, M. A. Berliner, C. J. Jewell and K. T. Kuwata, Chlorophyll Fluorescence Measurements to Assess the Competition of Substituted Anthraquinones for the Qb Binding-Site Biochim. Biophys. Acta 1990 1020 163–168
N. G. Bukhov, G. Sridharan, E. A. Egorova and R. Carpentier, Interaction of exogenous quinones with membranes of higher plant chloroplasts: modulation of quinone capacities as photochemical and non-photochemical quenchers of energy in Photosystem II during light-dark transitions Biochim. Biophys. Acta 2003 1604 115–123
D. Kirilovsky et al. Cytochrome c(550) in the cyanobacterium Thermosynechococcus elongatus - Study of redox mutants J. Biol. Chem. 2004 279 52869–52880
M. Seibert and M. R. Wasielewski, The isolated Photosystem II reaction center: first attempts to directly measure the kinetics of primary charge separation Photosynth. Res. 2003 76 263–268
A. M. Nuijs, H. J. Vangorkom, J. J. Plijter and L. N. M. Duysens, Primary-Charge Separation and Excitation of Chlorophyll-Alpha in Photosystem-II Particles from Spinach as Studied by Picosecond Absorbency-Difference Spectroscopy Biochim. Biophys. Acta 1986 848 167–175
J. A. Vanbest and P. Mathis, Kinetics of Reduction of Oxidized Primary Electron-Donor of Photosystem-II in Spinach-Chloroplasts and in Chlorella Cells in Microsecond and Nanosecond Time Ranges Following Flash Excitation Biochim. Biophys. Acta 1978 503 178–188
G. T. Babcock, R. E. Blankenship and K. Sauer, Reaction-Kinetics for Positive Charge Accumulation on Water Side of Chloroplast Photosystem 2 FEBS Lett. 1976 61 286–289
H. H. Robinson and A. R. Crofts, Kinetics of the Oxidation Reduction Reactions of the Photosystem-II Quinone Acceptor Complex, and the Pathway for Deactivation FEBS Lett. 1983 153 221–226
A. Zouni, H. T. Witt, J. Kern, P. Fromme and N. Krauss, Crystal structure of photosystem II form Synechococcus elongatus at 3.8 angstrom resolution Nature 2001 409 739–743
J. Haveman and P. Mathis, Flash-Induced Absorption Changes of Primary Donor of Photosystem-II at 820 nm in Chloroplasts Inhibited by Low pH or Tris-Treatment Biochim. Biophys. Acta 1976 440 346–355
G. Renger and C. Wolff, Existence of a High Photochemical Turnover Rate at Reaction Centers of System-2 in Tris-Washed Chloroplasts Biochim. Biophys. Acta 1976 423 610–614
A. Garbers, F. Reifarth, J. Kurreck, G. Renger and F. Parak, Correlation between protein flexibility and electron transfer from Q(A)? to Q(B) in PSII membrane fragments from spinach Biochemistry 1998 37 11399–11404
P. Faller et al. Rapid formation of the stable tyrosyl radical in photosystem II Proc. Natl. Acad. Sci. USA 2001 98 14368–14373
G. T. Babcock et al. Water Oxidation in Photosystem.2. From Radical Chemistry to Multielectron Chemistry Biochemistry 1989 28 9557–9565
B. Hillmann and E. Schlodder, Electron-Transfer Reactions in Photosystem-II Core Complexes from Synechococcus at Low-Temperature - Difference Spectrum of P680(+) Q(a)(−)/P680 Q(a) at 77 K Biochim. Biophys. Acta 1995 1231 76–88
L. K. Thompson and G. W. Brudvig, Cytochrome-B-559 May Function to Protect Photosystem-II from Photoinhibition Biochemistry 1988 27 6653–6658
S. Vasilév, G. W. Brudvig and D. Bruce, The X-ray structure of photosystem II reveals a novel electron transport pathway between P680, cytochrome b(559) and the energy-quenching cation, Chl(Z)(+) FEBS Lett. 2003 543 159–163
C. C. Moser and P. L. Dutton in Photosystem I: The plastocyanin: ferrodoxin oxidoreductase in photosynthesis, ed. J. H. Golbeck, Kluwer/Springer, New York, 2005, ch. 34
C. T. Yerkes, G. T. Babcock and A. R. Crofts, A Tris-induced change in the midpoint potential of Z, the donor to photosystem II, as determined by the kinetics of the back reaction FEBS Lett. 1983 158 359–363
I. Vass and S. Styring, Ph-Dependent Charge Equilibria between Tyrosine-D and the S-States in Photosystem 2. Estimation of Relative Midpoint Redox Potentials Biochemistry 1991 30 830–839
R. Edge, E. J. Land, D. J. McGarvey, M. Burke and T. G. Truscott, The reduction potential of the beta-carotene?+/beta-carotene couple in an aqueous micro-heterogeneous environment FEBS Lett. 2000 471 125–127
D. H. Stewart and G. W. Brudvig, Cytochrome b(559) of photosystem II Biochim. Biophys. Acta 1998 1367 63–87
W. A. Cramer and J. Whitmarsh, Photosynthetic Cytochromes Annu. Rev. Plant Physiol. Plant Mol. Biol. 1977 28 133–172
M. Roncel et al. Redox properties of the photosystem II cytochromes b559 and c550 in the cyanobacterium Thermosynechococcus elongatus J. Biol. Inorg. Chem. 2003 8 206–216
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor James Barber on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Moser, C.C., Page, C.C. & Dutton, P.L. Tunneling in PSII. Photochem Photobiol Sci 4, 933–939 (2005). https://doi.org/10.1039/b507352a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b507352a