-
PDF
- Split View
-
Views
-
Cite
Cite
Franziska Roth-Walter, Luis F Pacios, Rodolfo Bianchini, Erika Jensen-Jarolim, Linking iron-deficiency with allergy: role of molecular allergens and the microbiome, Metallomics, Volume 9, Issue 12, December 2017, Pages 1676–1692, https://doi.org/10.1039/c7mt00241f
Close - Share Icon Share
Abstract
Atopic individuals tend to develop a Th2 dominant immune response, resulting in hyperresponsiveness to harmless antigens, termed allergens. In the last decade, epidemiological studies have emerged that connected allergy with a deficient iron-status. Immune activation under iron-deficient conditions results in the expansion of Th2-, but not Th1 cells, can induce class-switching in B-cells and hampers the proper activation of M2, but not M1 macrophages. Moreover, many allergens, in particular with the lipocalin and lipocalin-like folds, seem to be capable of binding iron indirectly via siderophores harboring catechol moieties. The resulting locally restricted iron-deficiency may then lead during immune activation to the generation of Th2-cells and thus prepare for allergic sensitization. Moreover, iron-chelators seem to also influence clinical reactivity: mast cells accumulate iron before degranulation and seem to respond differently depending on the type of the encountered siderophore. Whereas deferoxamine triggers degranulation of connective tissue-type mast cells, catechol-based siderophores reduce activation and degranulation and improve clinical symptoms. Considering the complex interplay of iron, siderophores and immune molecules, it remains to be determined whether iron-deficiencies are the cause or the result of allergy.
Atopic individuals are often iron-deficient and tend to develop a Th2 dominant immune response, resulting in hyperresponsiveness to harmless antigens, termed allergens.
Introduction
Iron is an essential nutrient utilized in almost every aspect of normal cell function. All cells require iron to proliferate, iron being essential for DNA biosynthesis, protein function and cell cycle progression. In humans, iron is critical for a wide variety of biological processes as it allows transportation of oxygen, aids in the energy household and is essential for a healthy immune system.
Iron deficiency is probably the most common cause for anaemia, which by clinical definition is an insufficient mass of circulating red blood cells, and in public health terms is defined as a haemoglobin concentration below the thresholds given by the WHO, UNICEF and UNU.1,2 As such, iron deficiency can exist in the absence of anaemia, if it is mild or if the deficiency lasts not long enough.2
Iron deficiency can be absolute or functional, though combinations also exist. In absolute iron deficiency, the body has to cope with increased iron demand (e.g. during growth, blood donations, bleedings, and infections) that cannot sufficiently be compensated by dietary iron absorption and the release of recycled iron from senescent erythrocytes by macrophages.3 In contrast, during functional iron deficiency, the body has enough iron stores in the form of iron-laden ferritin in the liver, spleen and bone marrow, but iron-trafficking is on hold and/or reversed.
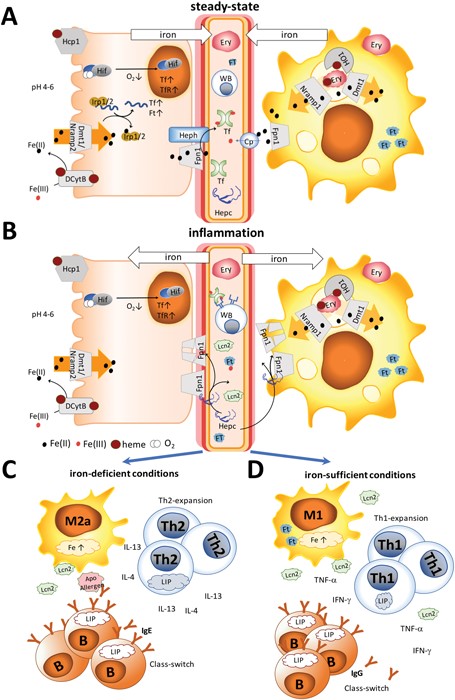
The different settings of iron metabolism are illustrated in Fig. 1. Under normal steady-state conditions, recycled iron is continuously released into the circulation by macrophages and dietary iron by enterocytes via the iron-exporter ferroportin in the form of Fe(ii) (Fig. 1A). Subsequently, ceruloplasmin or membrane-bound hephaestin oxidizes iron to Fe(iii) for transport via transferrin.4 Functional iron deficiency (Fig. 1B) is the consequence of an activated immune system that is triggered by danger signals derived from pathogens or damaged tissue and which results in a stop of the continuous iron release from enterocytes and macrophages and the accumulation of circulating iron into macrophages.
Iron homeostasis under steady-state and inflammation. (A) Under steady-state conditions, dietary iron absorption occurs via (1) the divalent metal-ion transporter 1 (Dmt1) upon reduction of ferric iron in its ferrous form by ascorbic acid or duodenal cytochrome b (Dcytb) and (2) the heme transporter (Hcp1) in the form of heme. Iron is released into the circulation by (3) macrophages recycling senescent erythrocytes with the help of heme oxygenase 1 (HO1) and dietary iron by enterocytes via the iron-exporter ferroportin (Fpn1) in the form of Fe(ii). Subsequently, ceruloplasmin (Cp) or membrane-bound hephaestin (Heph) oxidizes iron to Fe(iii) for transport via transferrin (Tf). Intracellularly iron concentrations are regulated by the iron regulatory proteins (Irp)1/2. Binding of these proteins to iron results in the transcription of transferrin and ferritin (Ft). Also, a decrease of oxygen can activate hypoxia-inducible factor (Hif), leading to transcription of Tf and the transferrin receptor (TfR). (B) Under inflammation, not only hepcidin (Hepc), but also innate proteins like lipocalin 2 (Lcn2) and ferritin are released into the circulation, Hepc binding to ferroportin (FpN1), thereby initiating its degradation and leading to cytoplasmic accumulation of iron. The immune response differs depending on the human's iron status. (C) Under limited iron supply Th2-, but not Th1-cells, will expand due to their larger intracellular iron pool and secrete IL-4 and IL-13. M2 macrophages will differentiate into an allergic subtype, further providing a Th2 environment. B cells expand via transferrin receptor-independent mechanisms and induce class-switching in the presence of IL-4 and IL-13 towards IgE. Apo-allergens may cause local iron-deprivation or interfere with the regulatory functions of Lcn2 and further modulate the immune-activation. (D) In contrast, under sufficient iron-supply, macrophages may release Lcn2, Th1 cells will expand, secreting IFN-γ and TNF-α, and B cells will generate IgG antibodies.
Discrimination between the two forms is complicated by the existence of various degrees and levels of absolute and functional iron deficiency and their smooth transition into anemia.3 Moreover, many proteins contributing to iron homeostasis are also innate proteins, e.g. ferritin, lactoferrin, and lipocalin 2, which are released into the circulation upon immune activation to impede iron-sequestration by pathogens. This all complicates the assessment of the true iron status.
An interesting aspect of iron deficiency it that a decrease in red blood cells is often accompanied by a relative increase of the white blood cell population representing the immune cells per se.
With respect to allergy, several epidemiological studies have correlated a greater degree of iron deficiency in allergic subjects than in non-allergic individuals.5,6 Whether this is the result of an overboard in the immune response, in which a functional iron-deficiency becomes absolute, or whether an absolute iron-deficiency lays the ground for the generation of allergy remains to be determined.
This review aims to address several important environmental, immunological and physiological aspects that may influence an individual's iron homeostatic status and may contribute to the establishment of allergy.
Humans and iron
Redox chemistry of iron
Under physiological conditions, iron is largely found in the ferrous Fe(ii) or ferric Fe(iii) form and the metal's redox cycling properties make it highly suited to act as a biocatalyst in proteins or as an electron carrier. In general, Fe(iii) prefers oxygen ligands, while Fe(ii) favors nitrogen and sulfur ligands.7 In the human body typical examples of Fe(iii) binding proteins are lactotransferrin, transferrin and ferritin oxidizing Fe(ii) to Fe(iii) upon binding, and low molecular weight compounds in the blood also bind Fe(iii), with citric acid being the major representative.8 Also, amino acids, ATP/AMP, inositol phosphates and 2,5-dihydroxybenzoic acid have been described to chelate Fe(iii) but not Fe(ii).9 A different picture emerges in the cytosolic compartment of cells, in which about 1 μM of Fe(ii) predominates the labile iron pool, with glutathione in cellular concentrations ranging from 0.5 to 10 mM10,11 acting as a buffer9 and thus serving as a means for the subsequent incorporation of Fe(ii) into a wide range of iron-dependent enzymes and electron transfer proteins.9
Importantly, free redox-active iron can be very toxic under aerobic conditions due to the Haber–Weiss cycle.12 In this cycle Fe(iii) is reduced to Fe(ii) by superoxide or other reducing agents and the oxidation of Fe(ii) produces Fe(iii) and hydroxyl radicals. As such, iron serves as a catalyst and minute amounts of free iron are sufficient to produce significant levels of reactive oxygen species (ROS).
Human iron homeostasis
An 80 kg human body contains approximately 4 g of iron. Hemoglobin iron accounts for approximately 60% of total iron, the vast majority of which is found within circulating erythrocytes.13 Most of the remaining iron is stored in the liver within ferritin (≈1 g). From an immunological point of view, it is interesting to note that the next largest iron-stores are the macrophages (≈0.6 g) in the spleen, liver and bone marrow.14 Around 0.3 g of iron is stored as myoglobin of muscles. All other cellular iron-containing proteins and enzymes are estimated to bind a total of ≈8 mg of iron. Iron is delivered to most tissues via circulating transferrin, which carries ≈2 mg of this metal in the steady state. Iron mass in the total extracellular fluid volume is about 10 mg, implicating that the transferrin iron pool turns over several times a day.15 In healthy men plasma iron turnover ranges from 25 to 35 mg16 per day, of which only 5 to 10% is provided by absorption of dietary iron in the gut, and the rest is predominantly iron recycled from monocytes and macrophages of the liver, adipose tissue, bone marrow, spleen and lymph nodes.14
Nutritional iron uptake
Dietary iron requirements, as well as bioavailability, are mainly determined by an individual's iron homeostatic status, affected by physiological conditions and reflected to a large extent in serum hepcidin levels.17 The chief area of iron absorption is the duodenum and the proximal jejunum.18 The duodenum has some unique characteristics as its pH is more acidic, with a pH ranging from 4 to 5 compared to the rest of the small intestine that has a pH-range between 7 and 9, and is the site where pancreatic juices and bile enter the small intestine. Depending on its form, iron will also be transported,
(a) as heme (from meat) into the enterocytes via the high-affinity folate transporter, which is also the intestinal heme transporter PCP/HCP1 (SLC46A1).19–21 Interestingly, the duodenal cytochrome b, Dcytb, is also able to bind on the lumen and on the cytoplasmic side to heme molecules,22–25 though the implications of these binding sites have so far been investigated only for the cytoplasmic heme binding site.26
(b) as non-heme iron, typically through low molecular weight chelates of ferric iron, which can derive from meat or plants. After reduction by ascorbic acid and/or duodenal cytochrome b, Dcytb,24,25 iron enters the cells (enterocytes, macrophages, T cells) via the divalent metal-ion transporter 1, DMT1, pathway.26
(c) via other uptake-pathways that seem to exist – e.g. bile itself in “premicellar” concentrations has been shown to interact with iron(ii)27 and contribute to iron absorption.28,29 Iron-carrying proteins like ferritin from food are efficiently absorbed without depending on reduction or the heme transporter via receptor-mediated, clathrin-dependent endocytosis.30 Absorption and increased iron accumulation were also found in the liver, when Fe(ii) was ingested with glycine and asparagine, but not with other amino acids.31
Once in the cell, iron is exported by ferroportin 1, also known as IREG1, MTP1, SLC40A1, FPN1, and HFE4, into the circulation.32 In general, iron excretion is suppressed by iron deficiency and anaemia and enhanced during erythropoiesis and hypoxia.26
Known inhibitors of bioavailability for non-heme iron are phytates, which are inositol polyphosphates found predominantly in nuts, seeds and grains that form insoluble precipitates with iron,33 and polyphenolic compounds. Many of these fruit- and plant-derived polyphenols bind with high affinity to iron34 and can greatly affect iron homeostasis,35,36 as upon consumption, the plasma concentration of polyphenols can commonly reach values about 1–10 μM.37 In most studies, the consumption of large quantities of purified polyphenols has been reported to decrease the volunteers’ iron status. However, under more natural circumstances, it is reasonable to assume that polyphenols will be present in the food matrix already complexed with iron. As such, one study demonstrated that, while purified polyphenols decreased the iron parameters in the subjects, ingestion of polyphenols in context with iron significantly improved the iron and redox status in vivo.38
Regulation of iron homeostasis
Hepcidin, a 25 amino acid-long peptide, is the major regulator of iron homeostasis. It is mainly expressed in the liver, but can also be produced by parietal cells of the stomach39 and by macrophages.
As schematically presented in Fig. 1A, under steady-state conditions only a low concentration of hepcidin is present in circulation, with a median concentration of 7.8 nM found in men and 4.1 nM vs. 8.5 nM, in pre- and post-menopausal women, respectively.40 Dietary and recycled iron is continuously released into the circulation to meet the daily iron requirements of the human body. Excretion of ferrous iron into the circulation is mediated by ferroportin, and subsequently, ceruloplasmin or membrane-bound hephaestin oxidizes Fe(ii) to Fe(iii) for further transport via transferrin.
During inflammation (Fig. 1B), iron is removed from the circulation, mainly by upregulation of hepcidin, and many innate proteins like Lcn2 and ferritin are also secreted into the circulation to sequester iron. Importantly, also hepcidin-independent processes have been described in humans with iron-deficiency.40 Hepcidin binds to the iron-exporter ferroportin, leading to its degradation, while iron is retained within the cells.40,41 Thus, increasing body iron levels cause increased hepcidin expression, resulting in increased macrophage and liver cell iron sequestration, and decreased dietary iron absorption; the result is a reduction in serum iron.42 In contrast, decreasing body iron levels cause decreased hepcidin expression, resulting in an increased release of macrophage iron and accelerated dietary iron absorption.
Under absolute iron deficiency, a decreased hepcidin concentration will result in the release of the remaining iron stores and in an increase in the dietary iron absorption. Intracellular iron is also regulated by iron regulatory proteins (IRPs) 1 and 2 with iron-responsive elements, in which upregulation of these proteins reflects low body iron stores and an increase of dietary iron absorption.43 Moreover, oxygen can regulate iron homeostasis. During hypoxia, the hypoxia-inducible factors (HIF) can target genes encoding transferrin and the transferrin receptor, leading to increased expression of transferrin and thus increased transport of ferric iron into cells.44
Non-transferrin bound iron and the labile iron pool
Excreted iron is usually bound to transferrin, ferritin or heme. In the circulation, also non-transferrin-bound iron, NTBI, is present, which represents a heterogeneous population that comprises organic anions with low affinity to iron (e.g. citrate, phosphates and carboxylates), polyfunctional ligands (chelates, siderophores and polypeptides), albumin45 and surface components of membranes (e.g. glycans and sulfonates) able to bind Fe(ii) and Fe(iii). The NTBI is typically present in concentrations up to 10 μM and its existence has been correlated with high levels of transferrin saturation. Similarly, intracellularly, this heterogeneous population of redox-active Fe complexes is called the labile iron pool, LIP. In cells, the iron concentration usually ranges from 20 to 100 μM and is largely associated with proteins, whereas only a minor fraction of the cellular iron is present as the LIP (>1%, up to 5 μM46). In the cytosol, Fe(ii) is prevalent, with glutathione probably acting as a major buffer. The LIP is primarily found in erythroid and myeloid cells, as well as in neuronal cells.
Importantly, though the NTBI or LIP represents only a fraction of the total intra- and extracellular iron, fine-tuning the LIP-levels has physiologically and immunologically wide-reaching consequences. Both iron forms are the immediate therapeutic targets of diseases associated with a misbalance in the iron homeostasis, e.g. hereditary hemochromatosis, myelodysplastic syndromes and sickle-cell disease.47
The immune system
The immune system and iron
Iron is essential for many peroxide- and nitrous oxide-generating enzymes,48 and regulates cell growth, cell differentiation, and cytokine production. Iron can activate protein kinase C, which leads to phosphorylation of compounds regulating cell proliferation. In addition, iron is necessary for myeloperoxidase activity to form hydroxyl radicals, enabling neutrophils to efficiently eliminate bacteria.49 Thus, any misbalance in the iron homeostasis towards either deficiency or overload has wide-reaching immunological consequences.
Macrophages are immune cells known to store iron. The reason for the high presence of iron in macrophages is their pivotal role in systemic iron recycling, where senescent erythrocytes are cleared by predominantly splenic macrophages.50 In addition to their important role in erythrocyte clearance and maintenance, their anti-inflammatory or inflammatory state seems to be dependent on the iron content. Anti-inflammatory macrophages have a lower iron-content and an increased expression of proteins associated with iron efflux and can be discriminated from inflammatory macrophages, which harbour high iron-levels.51 Macrophage phagocytosis is generally unaffected by iron deficiency, even though macrophage bactericidal activity is affected.48 Neutrophils under iron-limited conditions show impaired or lower killing activity due to the reduced activity of myeloperoxidase and reduced mobility to inflamed sites. Likewise, NK-cells exhibit lower activity due to reduced differentiation and proliferation under iron-restricted conditions. Importantly, T lymphocytes can also actively modulate the NTBI pool by uptake and export, with T cell deficiency associated with an accumulation of iron in the liver and pancreas.52 Impaired iron-uptake via transferrin receptor 1 (TfR1) caused by mutations can result in severe B- and T-cell deficiencies due to the lack of activation, which can partly be compensated through the internalization of iron via NTBI-pathways.53
Allergy and iron
Allergy is an immune-mediated disease, caused by an aberrant immune response towards exogenous, normally harmless antigens derived from pollen, house dust mites, animal dander, insect venom and food components. In particular, in the westernized world the prevalence of allergy seems to increase, with almost 20% of the adults in Germany having at least one self-reported doctor diagnosed allergy.54 Also in the United States, the prevalence of respiratory allergies is approximately 20%, whereas the prevalence of food allergies has increased from 3 to 5% and the prevalence of skin allergies from 7 to 12%.55 Allergy defined as type I hypersensitivity causes an immediate reaction usually within minutes upon re-exposure to the antigen in an individual with preformed antigen-specific IgE antibodies. Allergic symptoms may affect the eyes, nose, skin, the lungs or the gastrointestinal tract, leading to red eyes, an itchy rash, runny nose, shortness of breath, swelling or diarrhea. In severe cases, a systemic reaction can occur, resulting in an anaphylactic shock.
Mechanistically, it is important to note that the presence of specific IgE antibodies, referred to as allergic sensitization, is a precondition of allergic symptoms. They are a product from plasma cells when stimulated by T-helper 2 cell (Th2-) cytokines IL-4 and IL-13. A Th2 bias in the immune response is typically associated with allergies. Re-exposure to the allergen results in binding and cross-linking of IgE antibodies bound via high-affinity receptors on effector cells. Mast cells then discharge their granules containing histamine, leukotrienes and other active agents by exocytosis, causing allergic reactions.56 However, many people may just be sensitized, thus already having IgE-antibodies, but not yet an allergic reaction upon exposure to the respective allergen.54
As such, the events leading to the initiation of IgE formation are poorly understood, though it is generally accepted that the shift in the immune balance towards Th2 directly correlates with the overproduction of allergen-specific IgE.
Epidemiological and experimental evidence
Iron and allergy
The poor iron status at birth was associated with an increased risk of developing allergic diseases.57 It has been argued that a massive perinatal lymphocyte expansion may have led to a prioritization of erythrocytes at the expense of other vital developing tissues58 due to the restricted iron supply.57 This may then have compromised vulnerable Th1 lymphocytes having lower intracellular iron stores and may have promoted eosinophilia. Accordingly, when the maternal iron status during pregnancy was reduced, this was adversely associated with childhood wheezing, altered lung function and atopic sensitisation in the first 10 years of life.59
Along with a lower iron status increasing the risk of atopy, high iron concentration in umbilical cord samples was associated with a decreased risk of wheezing and eczema in the population-based Avon Longitudinal Study of Parents and Children.60 Similarly, in a British study decreased serum ferritin levels were found in children with atopic eczema.61 The lower iron-status is a consistent and a reproducible finding in multiple US cohorts, which clearly associates atopy with anemia.5 Also in a case-controlled, population-based Korean study, low beta-carotene, iron, folic acid, and dietary vitamin E were associated with atopic dermatitis in young children.62 Another study showed a greatly reduced incidence of wheezing and asthma in infants of mothers who were supplemented during pregnancy with vitamin C, a known contributor to increasing iron bioavailability.63 The change in the iron status impacted allergy also in vivo in a murine model of allergic asthma, in which oral iron supplementation, as well as systemic iron administration, suppressed airway manifestations.64
Misregulated iron-metabolism can affect atopy or the generation of allergies. Patients with a much too high iron load due to frequent blood transfusions have a decreased CD4/CD8 ratio,65,66 and their increased serum ferritin levels significantly correlate with the number of transfusions.
More female allergy sufferers: association with iron?
Finally, as iron homeostasis differs between the genders67 and before and after adolescence, it may contribute to the differences seen in the prevalence of allergy in the various groups. During childhood, boys are more affected by allergy than girls, but this changes in adulthood and women are more likely to be affected than men. In a German evaluation, 24% of men, but 35% of women suffer from at least one allergy and 2.9% of men, but 6.4% of women suffer from food allergies.68 Over 20% of Portuguese women were found iron-deficient and other studies have also confirmed that females present more often iron deficiency.69,70
All in all, there is consistent evidence that the iron-status of allergic subjects is reduced and may be linked with the disease.
Iron and cancer
It should be considered that cancer is in general associated with a highly suppressed immune defense (tumor tolerance phenomenon) and thus cancer and allergy have opposite immunological features.71 In fact, epidemiological studies have suggested that an inverse association between allergic diseases and cancer exists, indicating that allergic and/or atopic subjects with elevated IgE levels have a higher risk for allergies, but a lower risk of developing certain cancer types.72 As far as the iron content is concerned, while decreased iron levels predispose to allergies, increased serum iron correlates with an increased cancer risk.73–78
Therefore, iron levels affect in opposite ways the immune regulation in allergy and cancer.
Immune effects of iron
Iron deficiency affects more Th1 than Th2 immune cells
The proliferative phase of T-cells is dependent on iron supply. Activation of T cells leads to the expression of TfR via an IL2-dependent pathway, which facilitates iron uptake. The iron availability is known to differentially modulate the proliferation of different Th cell subsets. Th1 cells usually associated with inflammation have a lower labile iron pool and activation of Th1 cells can be readily blocked79 by inhibiting iron-uptake via TfR and/or deferoxamine, whereas Th2 cells seem to maintain a larger amount of iron in an assumable less labile and less readily chelatable pool that is only partly affected by blocking TfR and/or deferoxamine.80 As such, under iron-restricted conditions, DNA synthesis in Th1, but not Th2 cells, was inhibited. In line with these experiments, the Th1-associated cytokines IFN-γ and the IL-12/IL-18 mediated proliferation was found to be severely affected by iron chelators, whereas Th2-associated cytokines IL-13, which is IL-4 mediated, were resistant to potent iron chelators.81
Thus, it is very likely that under conditions of lymphocyte expansion the limited iron supply in allergic subjects will favour the development of a Th2-environment, thereby preparing for later allergic sensitisation.
M2 macrophages are more susceptible to iron-deprivation
Classical activated M1 macrophages are characterized by secretion of inflammatory cytokines, harbour high iron-levels51 and are important contributors to the classical Th1 response. In contrast, alternative activated M2 macrophages usually residing in the tissues have immunoregulatory functions, have a lower iron-content and participate in Th2 reactions and in wound healing processes.
Corna et al. demonstrated that under iron deprivation by deferoxamine both M1 and M2 macrophages enhanced IRP1 activity, whereas IRP2 was more strongly enhanced in M2 macrophages.82 Deferoxamine treatment also does not lead to the suppression of ferritin heavy chain expression in M1 macrophages, indicating high enough iron stores, whereas in M2 macrophages TfR1 was upregulated for continuous iron supply.51 As such, M1 macrophages are not as affected by iron-deficient conditions as M2 macrophages. Importantly, M2 cells are not as efficient in expressing molecules involved in antigen presentation, such as MHC class II (I-Ab), or the costimulatory molecule CD86 after T-cell stimulation under iron-deficient conditions, whereas M1 macrophages seem unaffected by iron chelators.
Iron suppresses class-switching in B-cells
Resting B-cells express much lower levels of TfR on the plasma membrane than on T-cells83 and seem to have evolved also transferrin-independent iron uptake mechanisms.84 B-cell proliferation and plasmacytic differentiation are not affected by Fe(ii) although ferrous iron seems to inhibit activation-induced cytidine deaminase (AID), an important enzyme to initiate class-switching, in a dose-dependent and specific manner, while other bivalent ions such as Zn(ii), Mn(ii), Mg(ii), and Ni(ii) did not inhibit class-switching.85 As such, iron-deficiency may facilitate IgE class switching in the presence of Th2-associated cytokines like IL-4 and IL-13. Alternatively, B-cells can be stimulated by TGF-β to secrete IgA, and the iron-carrying protein lactoferrin is also able to directly interact with betaglycan (TGF-β receptor III, TβRIII) and initiate class-switching to IgA and IgG2b.86 Thus, though proliferation and plasmacytic differentiation are not affected,49 the AID enzyme responsible for class switching can be active under iron-deficient conditions.85
Flavonoids impair mast cell function
Mast cells are important effector cells in triggering IgE-mediated allergic reactions and impeding mast cell release is a strategy of allergy treatment. It is known that, after priming with IgE and antigens, mast cells accumulate Fe(iii)87 before degranulation. On the other hand, the iron chelator desferrioxamine can similarly activate connective tissue-type mast cells via non-IgE mediated mechanisms as compound 80/40, but fails to activate basophils and mucosal-type mast cells and does not elicit late phase reaction similar to compound 80/40. As such, desferrioxamine has been suggested as a positive control in intradermal skin tests.88 Still, many flavonoids such as apigenin, quercetin, luteolin, fisetin89 and epicatechin that are strong iron chelators (Fig. 2) have been shown to exhibit anti-allergic activity in vitro and in in vivo models.90,91 Luteolin inhibited mast cell activation,92 naringenin inhibited allergen-induced airway inflammation,93 baicalin suppressed food allergy by the generation of regulatory T-cells,94 cacao-bean extract containing catechins ameliorated house dust mite induced atopic dermatitis,95 rutin suppressed atopic dermatitis,96 afzelin ameliorated asthma,97 and proanthocyanidins, oligomers consisting of catechin, epicatechin and gallic esters,98 and apigenin99 inhibited airway inflammation. In double-blind placebo controlled clinical trials, O-methylated catechins reduced symptoms of Japanese cedar pollinosis100 and catechins also reduced symptoms in mild and moderate atopic dermatitis.101 From this, it becomes apparent that iron levels and chelators are able to regulate mast cells and thus have an impact on the severeness of allergic symptoms.
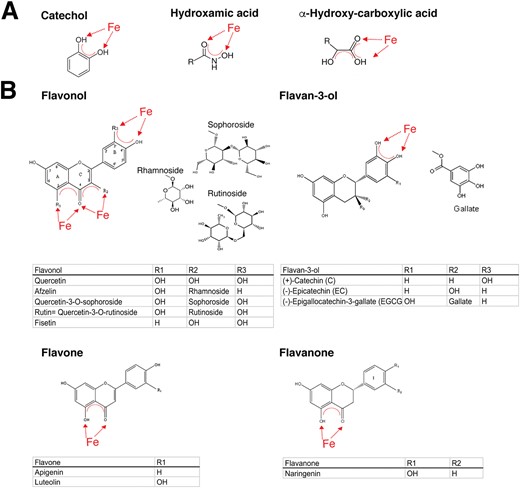
Structures with affinity to iron. (A) Siderophores can be classified by their ligands interacting with iron as catecholates, hydroxamates, and hydroxyl-carboxylates. (B) The four different subclasses of flavonoids namely flavonols, flavan-3-ols, flavones and flavanones, with described ligand-binding activity to allergens or described as having anti-allergic properties.
Allergens
Structure–function relationships: role for siderophores and iron
One of the fundamental riddles in allergy is why certain proteins emerge as allergens. It is assumed that they are directly related to the critical events triggering the Th2 bias. Despite the existence of thousands of protein families, the structures of major allergens can be restricted to few protein families. Although many allergens have been characterized in terms of secondary and tertiary structures, it is still uncertain whether common structural, functional or biochemical features underlie their ability to generate an allergic response.
Nearly all major allergens from mammals belong to the lipocalin family,102 while plant allergens usually originate from the prolamin (2S albumin, lipid binding proteins (LTPs)) and cupin (7S, 11S) superfamilies or from the pathogenesis-related (PR)-10 family.103 All members of these families share certain characteristics like their great structural stability and their ability to serve as carriers for a variety of compounds with lipidic segments.104,105
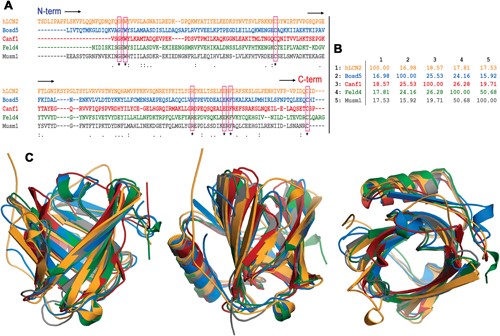
Allergens deriving from mammalian sources usually belong to the lipocalin family. Lipocalins show unusually low levels of overall sequence conservation with pairwise sequence identities often below 20%. Nevertheless, as illustrated in Fig. 3 the lipocalin fold is highly conserved.106 This β-barrel structure shapes a calyx-like site which gives the name to the protein family and is the main feature regarding the binding abilities of the lipocalin fold.107 While the wider end of the barrel is open to the solvent and rich in polar and charged amino acids, the narrower end is an inner, buried region rich in hydrophobic amino acids. Loops flanking the calyx display a great sequence variability that endows lipocalins with the ability to bind a large variety of ligands having polar and non-polar moieties. This property has been exploited in protein design that uses lipocalins as scaffolds to engineer novel binding proteins (“anticalins”).108
Sequence and structural alignment of lipocalins. (A) Clustal multiple sequence alignment of the following lipocalins. hLcn2: human Lcn2, Bos d 5: bovine β-lactoglobulin, Can f 1: dog allergen, Fel d 4: cat allergen, and Mus m 1: pheromone binding rodent major urinary protein from a mouse. The seven residues strictly conserved are boxed. (B) Percent identity matrix for the multiple sequence alignment in A. (C) Three views of the structural superposition of the hLcn2 (orange) crystal structure PDB id 1l6 m, Bos d 5 (blue) crystal structure PDB id 3NPO, Can f 1 (red) and Fel d 4 (green) homology model structures (ref. 100), and Mus m 1 (gray) crystal structure PDB id 1MUP. The percentages of allergen residues and RMSD values for backbone atoms in the structural superposition with hLcn2 are the following – Bos d 5: 67%, 1.362 Å; Can f 1: 66%, 1.574 Å; Fel d 4: 68%, 1.235 Å; Mus m 1: 73%, 1.158 Å.
Lipocalins are usually secreted and can be found in the dander, urine, fur, and saliva of animals.109 They have been described as powerful bacteriostatic agents against various microorganisms, by impeding their iron sequestration and binding to siderophore–iron complexes,110,111 but in accordance with their ligand binding plasticity, lipocalins also seem to act as carriers for lipids and hormones.
The human homologue lipocalin 2 (Lcn2, NGAL) has immune-regulatory function, but is also a growth factor112 and a stress protein that is released under various inflammatory conditions and in cancer.113 The binding of Lcn2 to bacterial siderophores, which are low molecular weight compounds that are amongst the strongest soluble Fe(iii) binding agents known, is considered a key feature against bacterial infections. Secreted Lcn2 seems to have specialized towards siderophores with catechol-moieties that facilitate their binding in the calyx site (see Fig. 4).114 Moreover, Lcn2 has been described to bind to endogenous siderophores like 2,5-dihydroxybenzoic acid (2,5-DHBA),115 thereby probably ensuring that excess free iron does not accumulate in the cytoplasm. Mammalian cells lacking this endogenous siderophore accumulate abnormally high levels of intracellular iron, leading to high levels of reactive oxygen species.115
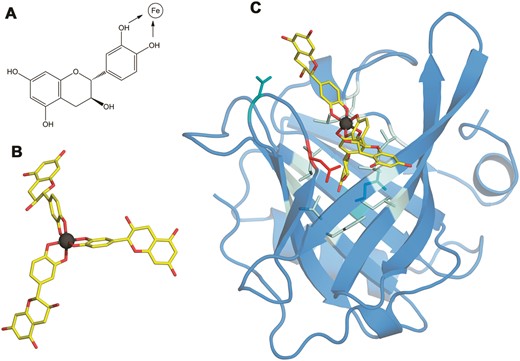
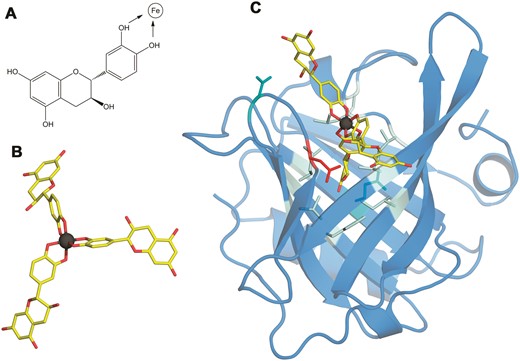
Structure of the Bos d 5–Fe(catechin)3 complex. (A) The structural formula of catechin. Catechol hydroxyls participating in the chelation of iron are marked with arrows. (B) 3D structure of the Fe(catechin)3 ligand showing the spatial arrangement of catechols to set the hexadentate octahedral coordination of iron (carbons in yellow, oxygen in red, and iron in dark gray). (C) 3D structure of the Bos d 5–Fe(catechin)3 complex showing the location of the ligand at the calyx of the lipocalin. Residues within a 3.5 Å distance from the ligand are displayed as sticks with the following color code: nine non-polar in pale cyan, two polar in cyan and one acidic in red.
Siderophores and iron modulate the Th2-potency of allergens
As iron is essential for almost all life, microbes and plants have evolved efficient iron sequestration strategies by producing siderophores that usually form a stable, hexadentate, octahedral complex predominantly with Fe(iii).116 Siderophores are usually classified by the ligands used to chelate the ferric iron with the major types of siderophores having catechol, hydroxamate or α-hydroxycarboxylate moieties, as depicted in Fig. 2. Also, combinations of the chemical groups are possible with microbes often employing non-ribosomal peptides to act as siderophores.117
Importantly, also many fruits and plants feature the presence of polyphenols/flavonoids known to behave as high-affinity iron chelators, a fact that is commonly overlooked,34 but that may contribute to their mast cell stabilizing ability. In addition, the ability of those compounds to chelate iron has been proposed to be a key element in their anti-oxidant properties.118
With regard to allergy, there seems to exist a particular role for catechol-type siderophores, which may also be related to the fact that catechol groups chelate iron119,120 at physiologically relevant pHs.
In this respect, it is relevant that lipocalin 2 can bind to only catechol-containing siderophores and not to others. This is an important characteristic that also seems to be extendable to many major allergens. Many lipocalin allergens such as the major milk allergen Bos d 5, or the major birch allergen Bet v 1, a prototype for the PR-10 protein family with a lipocalin-like architecture, are capable of transporting iron via catechol-type siderophores.121,122 Importantly, their loading state, apo- (empty) or holo- (filled), seemed to be decisive for the subsequent immune response. Apo-allergens can mount a Th2-response in vitro, whereas the holo forms are rather immune-suppressive, indicating that the iron-carrying form impedes allergic sensitization.121,122
As such, the natural ligand of the major birch pollen allergen Bet v 1 has been identified to be the flavonoid quercetin-3-O-sophoroside;123 the major peanut allergens Ara h 2 and Ara h 6, belonging to the 2S family, bind the flavonoid epigallocatechin-3-gallate;124 the major peanut allergen Ara h 1 from the 7S family forms large complexes by binding proanthocyanidins, which are oligomers consisting of catechin and epicatechin and their gallic acid esters.125 Accordingly, the pathogenesis-related PR-10 proteins and major allergens in strawberries, Fra a 1 and Fra a 3, have been crystallized with catechin ligands.126
In summary, there is solid evidence that allergens are capable of binding in particular catechol-type structures like quercetin and rutin with high iron affinities that surpass iron-affinity constants of deferoxamine.127,128
Importantly, a great number of these polyphenols have been described in the literature to exhibit anti-allergic properties. Taking lipocalins as an example, allergens are not only structurally at the border between self and non-self, but may also functionally interfere with the human immune-regulatory processes.
How normally harmless antigens become allergens with their characteristic Th2 skewing capacity is not known. There are several possible scenarios: (i) iron deficiency turns holo-lipocalins into apo-allergens, or (ii) exogenous lipocalins enter the body already devoid of a ligand in their calyx. In this case, they – due to their high affinity – will immediately sequester endogenous siderophores or comparable ligands directly at the mucosal sites, thereby contributing locally to an iron-deficient state. Additional triggers then may activate lymphocytes to become Th2 rather than Th1 cells.121,122,128 (iii) Allergenic lipocalins may in an ongoing immune response interfere with the function of Lcn2, e.g. during infections either by providing or by sequestering ligands for Lcn2. In any of these cases, the immune function of Lcn2 may be skewed by allergens towards Th2.
Can microbiota interfere with iron homeostasis?
From hygiene hypothesis to colonizing microbiota
The so-called hygiene hypothesis originated from observations that hay fever is more common in small families having few members than in large families and is associated with a less “dirty” environment, hence less microbial exposure.129 Moreover, living on a farm protects against atopy, hay fever, and asthma, especially in children.130 As such, cumulative evidence suggests that decreased exposure to microorganisms promotes allergy. The microbiota that colonizes humans has not merely a commensal, but rather a mutualistic relationship with their human host. It is accepted today that as such the bacterial composition has a profound impact on the immune system and vice versa.
Facts on microbiota in healthy versus allergic subjects
In the human healthy gut over 1000 bacterial species have been described,131 with an individual having at least 160 species, of which 52 species account for over 90%.132 Aerobic organisms including Streptococci and Lactobacilli, occasionally with Candida spp., predominantly colonize the human stomach, duodenum, and proximal jejunum,133 whereas an anaerobic predominance prevails in the distal ileum and colon. Dominant colonic organisms in humans include Clostridium spp., Bacteroides and Bifidobacterium.133 Thus, the Firmicutes and Proteobacteria phyla predominate in the duodenum, whereas Firmicutes and Bacteroidetes are the predominant phyla in the distal colon.
Microbiome depletion as a result of broad-spectrum antibiotic treatment led not only to anaemia and thrombocytosis but also to an altered immune homeostasis, resulting in diminished granulocytes and B cells116 in the bone marrow and increased CD8+ T-cells in mice. Importantly, faecal microbiota transfer partly rescued these hematopoietic changes.134 In rats inoculated with human faecal microbiota, provision of an iron-fortified diet after feeding an iron-deficient diet significantly increased the abundance of dominant bacterial groups such as Bacteroides spp. and Clostridium cluster IV members compared with rats on an iron-deficient diet only. Moreover, iron supplementation increased the gut microbial butyrate concentration 6-fold compared with iron depletion and did not affect histological colitis scores, suggesting that iron supplementation enhanced the concentration of beneficial gut microbiota metabolites and may contribute to gut health.135
Importantly, microbes able to inhabit the upper G/I tract seemed to be reduced in allergic subjects compared to non-allergic subjects.
There are only a few studies on the microbiota conducted in humans. As such, food allergic patients seem to have an increased abundance of bacteria of the order Clostridiales (Lachnospiraceae, Ruminococcaceae)136–139 and a decreased abundance of the order Bacteroidales.136,140,141
Microbiota interfere with iron levels via siderophores
An interesting aspect here is that Proteobacteria, Bacteroidetes and some family members of Firmicutes (see Table 1) are more likely to influence dietary iron uptake in the host due to the fact that their site of residency coincides with the site of iron-uptake. When screening these bacteria for their ability to secrete or utilize siderophores, it is apparent that indeed most of these organisms can acquire iron by siderophore-mediated mechanisms. As no data indicated the increased or decreased abundance of certain bacterial order, one can only speculate on their impact on iron homeostasis and on the immune cells.
Organisms in the human microbiota associated with allergy
| Phylum . | Family . | Genus . | Siderophore use . | Reported abundancy in allergy . |
|---|---|---|---|---|
| Actinobacteria137 | Bifidobacteriaceae136,140,141 | Bifidobacterium140 | +/−, Fe(ii) | Increased140/decreased136–138,141 |
| Coriobacteriaceae140 | Collinsella140 | + | Increased140/decreased137,141 | |
| Bacteriodetes137,140,142 | Bacteroidaceae | Bacteroides | +/hr | Decreased137,140,141 |
| Rikenellaceae | Rickenella | +/heme | Decreased141 | |
| Porphyromonadaceae | Porphyromonas | Fe(ii)/heme | ||
| Parabacteroides140 | ? | Decreased140, 141 | ||
| Prevotellaceae | Prevotella | +, heme, tr − | Decreased140, 141, 143 | |
| Firmicutes | Lactobacillaceae | Lactobacillus140,141 | −/Mn | Increased140 |
| Streptococcaceae | Streptococcus140 | +, fr, lr, heme, hr | Decreased138,140,141 | |
| Enterococcaceae | Enterococcus | +, heme | Decreased138 | |
| Staphylococcaceae | Staphylocoocus | + | Decreased138,145 | |
| Clostridiaceae/cluster I140,141 | Clostridium | +, heme | Increased136,137,141 | |
| Clostridiaceae/cluster IV140,141 | Butyricicoccus | ? | n/a | |
| Anaerobacter140 | ? | Increased137,140/decreased139 | ||
| Lachnospiraceae138,140,143,146,147 | Lactonifactor | ? | n/a | |
| Blautia | + | Decreased143/increased138 | ||
| Roseburia | + | Increased138,140,141 | ||
| Ruminococcaceae/cluster IV140,143 | Ruminococcus | + | Increased138,140,141 | |
| Faecalibacterium | + | Increased138,140/decreased139,141,143 | ||
| Subdoligranulum | Fe(ii) | Increased138,140/decreased141,143 | ||
| Veillonellaceae | Dialister141 | + | Decreased141 | |
| Megamonas | + | n/a | ||
| Megasphaera141 | + | Decreased141 | ||
| Veillonella140 | + | Decreased140,141 | ||
| Proteobacteria137 | Enterobacteriaceae139 | Escherichia | + | Decreased138 |
| Citrobacter | + | Increased139 | ||
| Pseudomonadaceae | Pseudomonas | + | ||
| Moraxellaceae | Acinetobacter144 | +148 | Decreased144 | |
| Campylobacteraceae | Campylobacter149 | + | Decreased150 | |
| Arcobacter | + | |||
| Verrucomicrobia | Akkermansiaceae140 | Akkermansia | + | Increased140/decreased139,141 |
| Kingdom Fungi: Ascomycota | Saccharomycetaceae | Candida | + | Increased141 |
| Phylum . | Family . | Genus . | Siderophore use . | Reported abundancy in allergy . |
|---|---|---|---|---|
| Actinobacteria137 | Bifidobacteriaceae136,140,141 | Bifidobacterium140 | +/−, Fe(ii) | Increased140/decreased136–138,141 |
| Coriobacteriaceae140 | Collinsella140 | + | Increased140/decreased137,141 | |
| Bacteriodetes137,140,142 | Bacteroidaceae | Bacteroides | +/hr | Decreased137,140,141 |
| Rikenellaceae | Rickenella | +/heme | Decreased141 | |
| Porphyromonadaceae | Porphyromonas | Fe(ii)/heme | ||
| Parabacteroides140 | ? | Decreased140, 141 | ||
| Prevotellaceae | Prevotella | +, heme, tr − | Decreased140, 141, 143 | |
| Firmicutes | Lactobacillaceae | Lactobacillus140,141 | −/Mn | Increased140 |
| Streptococcaceae | Streptococcus140 | +, fr, lr, heme, hr | Decreased138,140,141 | |
| Enterococcaceae | Enterococcus | +, heme | Decreased138 | |
| Staphylococcaceae | Staphylocoocus | + | Decreased138,145 | |
| Clostridiaceae/cluster I140,141 | Clostridium | +, heme | Increased136,137,141 | |
| Clostridiaceae/cluster IV140,141 | Butyricicoccus | ? | n/a | |
| Anaerobacter140 | ? | Increased137,140/decreased139 | ||
| Lachnospiraceae138,140,143,146,147 | Lactonifactor | ? | n/a | |
| Blautia | + | Decreased143/increased138 | ||
| Roseburia | + | Increased138,140,141 | ||
| Ruminococcaceae/cluster IV140,143 | Ruminococcus | + | Increased138,140,141 | |
| Faecalibacterium | + | Increased138,140/decreased139,141,143 | ||
| Subdoligranulum | Fe(ii) | Increased138,140/decreased141,143 | ||
| Veillonellaceae | Dialister141 | + | Decreased141 | |
| Megamonas | + | n/a | ||
| Megasphaera141 | + | Decreased141 | ||
| Veillonella140 | + | Decreased140,141 | ||
| Proteobacteria137 | Enterobacteriaceae139 | Escherichia | + | Decreased138 |
| Citrobacter | + | Increased139 | ||
| Pseudomonadaceae | Pseudomonas | + | ||
| Moraxellaceae | Acinetobacter144 | +148 | Decreased144 | |
| Campylobacteraceae | Campylobacter149 | + | Decreased150 | |
| Arcobacter | + | |||
| Verrucomicrobia | Akkermansiaceae140 | Akkermansia | + | Increased140/decreased139,141 |
| Kingdom Fungi: Ascomycota | Saccharomycetaceae | Candida | + | Increased141 |
Code: predominantly found in colon (bold face), upper G/I (regular font). Ferritin – fr, transferrin – tr, lactoferrin – lr, hemophores – hr.
Organisms in the human microbiota associated with allergy
| Phylum . | Family . | Genus . | Siderophore use . | Reported abundancy in allergy . |
|---|---|---|---|---|
| Actinobacteria137 | Bifidobacteriaceae136,140,141 | Bifidobacterium140 | +/−, Fe(ii) | Increased140/decreased136–138,141 |
| Coriobacteriaceae140 | Collinsella140 | + | Increased140/decreased137,141 | |
| Bacteriodetes137,140,142 | Bacteroidaceae | Bacteroides | +/hr | Decreased137,140,141 |
| Rikenellaceae | Rickenella | +/heme | Decreased141 | |
| Porphyromonadaceae | Porphyromonas | Fe(ii)/heme | ||
| Parabacteroides140 | ? | Decreased140, 141 | ||
| Prevotellaceae | Prevotella | +, heme, tr − | Decreased140, 141, 143 | |
| Firmicutes | Lactobacillaceae | Lactobacillus140,141 | −/Mn | Increased140 |
| Streptococcaceae | Streptococcus140 | +, fr, lr, heme, hr | Decreased138,140,141 | |
| Enterococcaceae | Enterococcus | +, heme | Decreased138 | |
| Staphylococcaceae | Staphylocoocus | + | Decreased138,145 | |
| Clostridiaceae/cluster I140,141 | Clostridium | +, heme | Increased136,137,141 | |
| Clostridiaceae/cluster IV140,141 | Butyricicoccus | ? | n/a | |
| Anaerobacter140 | ? | Increased137,140/decreased139 | ||
| Lachnospiraceae138,140,143,146,147 | Lactonifactor | ? | n/a | |
| Blautia | + | Decreased143/increased138 | ||
| Roseburia | + | Increased138,140,141 | ||
| Ruminococcaceae/cluster IV140,143 | Ruminococcus | + | Increased138,140,141 | |
| Faecalibacterium | + | Increased138,140/decreased139,141,143 | ||
| Subdoligranulum | Fe(ii) | Increased138,140/decreased141,143 | ||
| Veillonellaceae | Dialister141 | + | Decreased141 | |
| Megamonas | + | n/a | ||
| Megasphaera141 | + | Decreased141 | ||
| Veillonella140 | + | Decreased140,141 | ||
| Proteobacteria137 | Enterobacteriaceae139 | Escherichia | + | Decreased138 |
| Citrobacter | + | Increased139 | ||
| Pseudomonadaceae | Pseudomonas | + | ||
| Moraxellaceae | Acinetobacter144 | +148 | Decreased144 | |
| Campylobacteraceae | Campylobacter149 | + | Decreased150 | |
| Arcobacter | + | |||
| Verrucomicrobia | Akkermansiaceae140 | Akkermansia | + | Increased140/decreased139,141 |
| Kingdom Fungi: Ascomycota | Saccharomycetaceae | Candida | + | Increased141 |
| Phylum . | Family . | Genus . | Siderophore use . | Reported abundancy in allergy . |
|---|---|---|---|---|
| Actinobacteria137 | Bifidobacteriaceae136,140,141 | Bifidobacterium140 | +/−, Fe(ii) | Increased140/decreased136–138,141 |
| Coriobacteriaceae140 | Collinsella140 | + | Increased140/decreased137,141 | |
| Bacteriodetes137,140,142 | Bacteroidaceae | Bacteroides | +/hr | Decreased137,140,141 |
| Rikenellaceae | Rickenella | +/heme | Decreased141 | |
| Porphyromonadaceae | Porphyromonas | Fe(ii)/heme | ||
| Parabacteroides140 | ? | Decreased140, 141 | ||
| Prevotellaceae | Prevotella | +, heme, tr − | Decreased140, 141, 143 | |
| Firmicutes | Lactobacillaceae | Lactobacillus140,141 | −/Mn | Increased140 |
| Streptococcaceae | Streptococcus140 | +, fr, lr, heme, hr | Decreased138,140,141 | |
| Enterococcaceae | Enterococcus | +, heme | Decreased138 | |
| Staphylococcaceae | Staphylocoocus | + | Decreased138,145 | |
| Clostridiaceae/cluster I140,141 | Clostridium | +, heme | Increased136,137,141 | |
| Clostridiaceae/cluster IV140,141 | Butyricicoccus | ? | n/a | |
| Anaerobacter140 | ? | Increased137,140/decreased139 | ||
| Lachnospiraceae138,140,143,146,147 | Lactonifactor | ? | n/a | |
| Blautia | + | Decreased143/increased138 | ||
| Roseburia | + | Increased138,140,141 | ||
| Ruminococcaceae/cluster IV140,143 | Ruminococcus | + | Increased138,140,141 | |
| Faecalibacterium | + | Increased138,140/decreased139,141,143 | ||
| Subdoligranulum | Fe(ii) | Increased138,140/decreased141,143 | ||
| Veillonellaceae | Dialister141 | + | Decreased141 | |
| Megamonas | + | n/a | ||
| Megasphaera141 | + | Decreased141 | ||
| Veillonella140 | + | Decreased140,141 | ||
| Proteobacteria137 | Enterobacteriaceae139 | Escherichia | + | Decreased138 |
| Citrobacter | + | Increased139 | ||
| Pseudomonadaceae | Pseudomonas | + | ||
| Moraxellaceae | Acinetobacter144 | +148 | Decreased144 | |
| Campylobacteraceae | Campylobacter149 | + | Decreased150 | |
| Arcobacter | + | |||
| Verrucomicrobia | Akkermansiaceae140 | Akkermansia | + | Increased140/decreased139,141 |
| Kingdom Fungi: Ascomycota | Saccharomycetaceae | Candida | + | Increased141 |
Code: predominantly found in colon (bold face), upper G/I (regular font). Ferritin – fr, transferrin – tr, lactoferrin – lr, hemophores – hr.
The microbiota strongly manipulates the immune system. It is tempting to speculate that the composition and localization of the commensal microbiota in allergic subjects may directly impact the homeostatic iron status of the host, but more studies need to be done.
Conclusions
There is a clear epidemiological connection between a poor iron status and allergy risk, especially in females. Of note, iron-deficient conditions seem to promote a Th2-environment, which is a prerequisite for allergy. Potential contributing factors are endogenous iron levels, allergens capable of binding to iron chelators, and likely a skewed microbiota in allergic subjects.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Austrian Science Fund FWF grant SFB F4606-B28 to EJJ.
References
Franziska Roth-Walter studied Chemistry and completed her PhD at the University of Vienna. She did her post-doctoral training in Immunology at the Medical University of Vienna, Austria, and the Mount Sinai School of Medicine, New York, USA. Since 2011 she has been working in the Interuniversity Messerli Research Institute in Vienna. She habilitated for Immunology in 2014 at the Medical University of Vienna. Her research is focused on understanding the mechanisms in mucosal immunity and allergy.
Luis F. Pacios is a physical chemist, obtained his PhD in Quantum Chemistry at the University Complutense of Madrid and completed his training in Clarkson University, New York, USA. Since 1987 he has been Associate Professor in the Technic University of Madrid where he currently teaches Protein Structure and Computational Structural Biology. His research focuses on the computational modeling of molecular structures and interactions in protein–ligand systems.
Rodolfo Bianchini is a senior scientist at the Interuniversity Messerli Research Institute in the Department of Comparative Medicine, Vienna, Austria. He obtained his PhD in Clinical and Experimental Pharmacology at the University of Perugia, Italy, and completed his post-doctoral training at the University of Perugia and the University of Salzburg in Austria. His research is primarily focused on modulating macrophage responses in allergy and cancer.
Erika Jensen-Jarolim, MD, immunologist, holds a professorship at the Medical University of Vienna, affiliated to the Interuniversity Messerli Research Institute where she directs the Department for Comparative Medicine. She is active on multiple boards and councils. She sees allergic patients and applies molecular allergy diagnosis. Her research focuses on immune mechanisms of molecular allergens and the immune regulation in allergen immunotherapy and cancer, in line with the concept of AllergoOncology.