Abstract
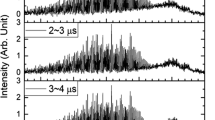
The photochemistry of the [(CpR)Mo(CO)3]2 molecules, where CpR = η5-C5H4(CH2)2C(O)NCH3(CH2)nCH3 (n = 3, 8, 13, and 18), was examined using femtosecond pump—probe transient absorption spectroscopy. The goal of this study was to investigate the importance of radical size and mass on the dynamics and efficiency of geminate radical—radical recombination. The femtosecond results demonstrated the lack of any size/mass dependence of the recombination efficiency. This finding contrasts with results from a prior study that did find a size/mass dependence using a steady-state photochemical technique. To explain these conflicting results, it is proposed that the femtosecond pump—probe results are a measurement of the efficiency of primary geminate recombination whereas the steady-state method results are a measurement of the sum of primary and secondary geminate recombination efficiencies. The size/mass dependence is evident in the latter because secondary geminate recombination is a slower diffusive recombination process and therefore depends on the steric properties of the radicals. Although the existence of primary and secondary recombination channels is often taken for granted, experimental differentiation of primary and secondary caging has proven to be difficult because it is not possible for a single experimental technique to span the entire timescale for recombination of a radical cage pair and adequately resolve these recombination pathways.
Similar content being viewed by others
Notes and references
T. Koenig and H. Fischer, in Free Radicals, ed. J. Kochi, John Wiley, New York, 1973, vol. 1,, ch. 4.
J. Franck, E. Rabinowitch Trans. Faraday Soc., 1934, 30, 120–131.
J. P. Lorand, The Cage Effect Prog. Inorg. Chem., 1972, 17, 207–325.
J. L. Male, B. E. Lindfors, K. J. Covert, D. R. Tyler, The effect of radical size and mass on the cage recombination efficiency of photochemically generated radical cage pairs J. Am. Chem. Soc., 1998, 120, 13176–13186.
A. E. Stiegman, D. R. Tyler, Photochemical disproportionation of metal–metal bonded carbonyl dimers Coord. Chem. Rev., 1985, 63, 217–240.
T. J. Meyer, J. V. Caspar, Photochemistry of metal–metal bonds Chem. Rev., 1985, 85, 187–218.
J. L. I. V. Hughey, C. R. Bock, T. J. Meyer, Flash photolysis evidence for metal-metal bond cleavage and loss of carbon monoxide in the photochemistry of bis[(η5-cyclopentadienyl) tricarbonylmolybdenum] J. Am. Chem. Soc., 1975, 97, 4440–4441.
A. R. Burkett, T. J. Meyer, D. G. Whitten, Light-catalyzed substitution and disproportionation in di-p-cyclopentadienylhexacarbonyldimolybdenum J. Organomet. Chem., 1974, 67, 67–73.
M. S. Wrighton, D. S. Ginley, Photochemistry of metal-metal bonded complexes. III. Photoreactivity, of hexacarbonylbis(η5-cyclopentadienyl)dimolybdenum(i) and -ditungsten(i) J. Am. Chem. Soc., 1975, 97, 4246–4251.
R. M. Noyes, A treatment of chemical kinetics with special applicability to diffusion-controlled reactions J. Chem. Phys., 1954, 22, 1349–1359.
B. E. Lindfors, J. L. Male, K. J. Covert, D. R. Tyler, Radical cage effects. Effect of radical mass and bond energies on cage recombination efficiencies for photochemical cage pair intermediates of [Mo2(CO)6(η5-C5H4CH2CH2OSiMe32], [Mo2(CO)6(η5-C5H4Me)2] and [W2(CO)6(η5-C5H4Me)2] Chem. Commun., 1997 1687–1688.
D. A. Braden, E. E. Parrack, D. R. Tyler, Solvent cage effects. I. Effect, of radical mass and size on radical cage pair recombination efficiency. II. Is, geminate recombination of polar radicals sensitive to solvent polarity? Coord. Chem. Rev., 2001, 211, 279–294.
E. Schutte, T. J. R. Weakley, D. R. Tyler, Radical cage effects in the photochemical degradation of polymers: effect of radical size and mass on the cage recombination efficiency of radical cage pairs generated photochemically from the (CpCH2CH2N(CH3)C(O)(CH2nCH32Mo2(CO)6 (n = 3, 8, 18) complexes J. Am. Chem. Soc., 2003, 125, 10319–10326.
K. J. Covert, E. F. Askew, J. Grunkemeier, T. Koenig, D. R. Tyler, Cage effects in organometallic radical chemistry. Fractional cage-recombination efficiency for photochemical caged-pair intermediates of Cp′2M2(CO)6 (M = molybdenum and tungsten; Cp′ = η5-C5H4CH3J. Am. Chem. Soc., 1992, 114, 10446–10448.
Q. Liu, J. K. Wang, A. H. Zewail, Femtosecond dynamics of dissociation and recombination in solvent cages Nature, 1993, 364, 427–430.
B. J. Schwartz, J. C. King, C. B. Harris, The molecular basis of solvent caging Understanding Chemical Reactivity, 1994, 7, 235–248.
B. J. Schwartz, J. C. King, J. Z. Zhang, C. B. Harris, Direct femtosecond measurements of single collision dominated geminate recombination times of small molecules in liquids Chem. Phys. Lett., 1993, 203, 503–508.
A. B. Oelkers, L. F. Scatena, D. R. Tyler, Femtosecond pump–probe transient absorption study of the photolysis of [Cp′Mo(CO)3]2 (Cp′ = η5-C5H4CH3); The role of translational and rotational diffusion in the radical cage effect J. Phys. Chem. A., 2007, 111, 25 5353–5360.
A. R. Manning, P. Hackett, R. Birdwhistell, P. Soye, Hexacarbonylbis(η5-cyclopentadienyl)dichromium, molybdenum, and tungsten and their analogs, M2(η5-C5H4R)2(CO)6 (M = Cr, Mo, and W; R = H, Me or PhCH2) Inorg. Syn., 1990, 28, 148–150.
D. A. Braden, E. E. Parrack, D. R. Tyler, Photochemical studies as a function of solvent viscosity. A new photochemical pathway in the reaction of (η5-C5H4Me)2Mo2(CO)6 with CCl4Photochem. Photobiol. Sci., 1, 418-420.
J. L. Male, B. E. Lindfors, K. J. Covert, D. R. Tyler, Cage effects in the photochemical degradation of polymers. Studies of model complexes with different chain lengths Macromolecules, 1997, 30, 6404–6406.
J. D. Harris, A. B. Oelkers, D. R. Tyler, The solvent cage effect: Is there a spin barrier to recombination of transition metal radicals? J. Am. Chem. Soc., 2007, 129, 6255–6262.
See ref. 4 and the supporting information therein.
B. A. Van Vlierberge, H. B. Abrahamson, Laser flash photolysis of bis[tricarbonyl(cyclopentadienyl)tungsten] under visible irradiation J. Photochem. Photobiol., A, 1990, 52, 69–81.
S. L. Scott, J. H. Espenson, Z. Zhu, Reactivity of 17-electron organometallic tungsten and molybdenum radicals: a laser flash photolysis study J. Am. Chem. Soc., 1993, 115, 1789–1797.
Q. Yao, A. Bakac, J. H. Espenson, Dissociation and recombination dynamics of the complexes [(η5-C5η5)Cr(CO)3]2 (R = hydrogen, methyl) Organometallics, 1993, 12, 2010–2012.
J. R. Knorr, T. L. Brown, Flash photolysis of molybdenum complex [(η5-C5H5)Mo(CO)3]2: mechanism of the formation and decay of the linear semibridged species ( η5-C5H52Mo2(CO)4(µ-η1,η2-CO) J. Am. Chem. Soc., 1993, 115, 4087–4092.
J. Peters, M. W. George, J. J. Turner, Photochemistry of [CpMo(CO)3]2. Direct detection and kinetics of the radical CpMo(CO)3 in n-heptane solution at room temperature by fast time-resolved infrared spectroscopy Organometallics, 1995, 14, 1503–1506.
J. C. Linehan, C. R. Yonker, R. S. Addleman, S. T. Autrey, J. T. Bays, T. E. Bitterwolf, J. L. Daschbach, Solvent study of the kinetics of molybdenum radical self-termination Organometallics, 2001, 20, 401–407.
J. L. Male, M. Yoon, A. G. Glenn, T. J. R. Weakley, D. R. Tyler, Radical cage effects in the photochemical degradation of polymers: In-cage trapping of photochemically generated radical cage pairs in polymer model compounds Macromolecules, 1999, 32, 3898–3906.
N. K. Szymczak, A. B. Oelkers, D. R. Tyler, Detection of hydrogen bonding in solution: A 2H nuclear magnetic resonance method based on rotational motion of a donor/acceptor complex Phys. Chem. Chem. Phys., 2006, 8, 4002–4008.
A. Tsutsumi, B. Perly, A. Forchioni, C. Chachaty, A magnetic resonance study of the segmental motion and local conformations of poly(l-glutamic acid) in aqueous solutions Macromolecules, 1978, 11, 977–986.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Additional kinetics data and vibrational relaxation time constants (s2) relevant to Table 1. See DOI: 10.1039/b709005f
Rights and permissions
About this article
Cite this article
Oelkers, A.B., Schutte, E.J. & David, D.R. Solvent cage effects: the influence of radical mass and volume on the recombination dynamics of radical cage pairs generated by photolysis of [CpCH2CH2N(CH3)C(O)(CH2)nCH3Mo(CO)3]2 (n = 3, 8, 13, 18) (Cp = η5-C5H4) complexes. Photochem Photobiol Sci 7, 228–234 (2008). https://doi.org/10.1039/b709005f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b709005f