Abstract
Preference choice on food is an important response strategy for animals living in the environment. Using assay system of preference choice on bacterial foods, OP50 and PA14, we identified the involvement of ADL sensory neurons in the control of preference choice in Caenorhabditis elegans. Both genetically silencing and ChR2-mediated activation of ADL sensory neurons significantly affected preference choice. ADL regulated preference choice by inhibiting function of G protein-coupled receptor (GPCR)/SRH-220. ADL sensory neurons might regulate preference choice through peptidergic signals of FLP-4 and NLP-10 and function of FLP-4 or NLP-10 in regulating preference choice was regulated by SRH-220. FLP-4 released from ADL sensory neurons further regulated preference choice through its receptor of NPR-4 in AIB interneurons. In AIB interneurons, NPR-4 was involved in the control of preference choice by activating the functions of ASH-2 trithorax complex consisting of SET-2, ASH-2 and WDR-5, implying the crucial role of molecular machinery of trimethylation of histone H3K4 in the preference choice control. The identified novel neuronal circuit and the underlying molecular mechanisms will strengthen our understanding neuronal basis of preference choice in animals.
Similar content being viewed by others
Introduction
Animals’ decision making includes two main levels of complexity, behavioral choice and value-based decision making1,2. Decision making may be helpful for maximizing the evolutionary fitness in an unpredictable world. Behavioral choice concerns proximal causes for the selection between alternative sensory cues2. Nematode Caenorhabditis elegans is an attractive model animal for behavioral study due to its simple nervous system and ability to sense diverse environmental stimuli, such as touch, smell, taste and temperature3,4. Simplicity and genetic tractability of C. elegans nervous system makes it a useful assay system for seeking the biological mechanisms of behavioral choice5,6. C. elegans can exhibit several types of behavioral choice based on its response to paired or multiple stimuli, such as attractants versus aversive stimuli or different attractants7,8,9,10,11,12.
In C. elegans, olfactory chemotaxis towards food-associated odors is an innate behavior and highly reproducible among animals13. Preference choice on bacterial food is one of the assayed behavioral choices in C. elegans12,14. In this behavioral choice assay system, nematodes will migrate towards one of the two examined bacterial lawns on opposite sides in a plate (Fig. 1a)12,14. Usually a harmless bacterium such as OP50 and a pathogenic bacterium such as PA14 are used in the preference choice assay system12,15. Previous study has implied that the deficits in differentiation of AWB or AWC sensory neurons, required for the olfactory perception, affect preference choice in nematodes14. In C. elegans, AWB and AWC sensory neurons are necessary for innate preference for Pseudomonas aeruginosa16 and neurotransmitters and neuropeptides used by AWB or AWC sensory neurons may control this innate odor preference17. Nevertheless, neuronal circuit involved in the control of preference choice on bacterial food is still largely unclear. In addition, only limited information is available for the molecular basis of preference choice on bacterial food in C. elegans.
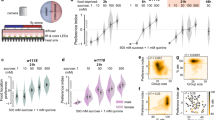
Preference choice on bacterial food in nematodes with genetically ablated or optogenetically activating ADL sensory neurons.
(a) Schematic representation of assay system for preference choice on bacterial food. (b) Preference choice on bacterial food in nematodes with genetically ablated ADL or ASH sensory neurons. (c) Preference choice on bacterial food in nematodes with optogenetical activation of ADL sensory neurons. Optogenetical activation was performed under the lite-1 mutation background. Control, lite-1(ce314). Bars represent means ± S.E.M. **P < 0.01 vs N2 (if not specifically indicated).
Among the well-known 12 kinds of amphid neurons (AWA, AWB, AWC, AFD, ASE, ADF, ASG, ASH, ADL, ASI, ASJ and ASK) in C. elegans, ASH and ADL are important sensory neurons functioning to sense and mediate avoidance of noxious environmental stimuli, such as Cu2+ ion and garlic18,19,20. In this study, using quantitative behavior assays and genetic and neuronal manipulations, we determined the possible roles of these sensory neurons in regulating preference choice on bacterial food and the underlying neuronal and molecular mechanisms in C. elegans. The new identified neuronal circuit and the related underlying mechanism for preference choice on bacterial food will strengthen our understanding neuronal basis of behavioral choice in animals.
Results
Genetically silencing ADL sensory neurons altered preference choice of nematodes on bacterial food
To identify candidate sensory neurons involved in the control of preference choice on bacterial food, we employed the strain with genetically silencing ASH or ADL sensory neurons. In the strain of ASH::TeTx, TeTx was specifically expressed in ASH sensory neurons using a FLP–FRT site-specific recombination system to permanently block the ASH neurotransmission20. egl-1 gene encodes a cell-death activator and can be used to genetically ablating specific neurons21. We obtained a strain in which ADL sensory neurons were genetically ablated by expressing egl-1 under the control of srh-220 promoter. Interestingly, genetically ablating ASH sensory neurons did not significantly affect preference choice compared with wild-type N2 (Fig. 1b). In contrast, we found that genetically ablating ADL sensory neurons significantly increased choice index in the preference choice assay system (Fig. 1b). Meanwhile, nematodes with genetically ablated ASH or ADL sensory neurons had similar leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 compared with wild-type N2 (Fig. S1).
Optogenetically activating ADL sensory neurons affected preference choice of nematodes on bacterial food
To further confirm the role of ADL sensory neurons in regulating preference choice, we performed optogenetic manipulation of ADL sensory neurons. All the optogenetic manipulations of ADL sensory neurons were performed under the lite-1 mutation background in order to eliminate the intrinsic photophobic response of C. elegans22. The lite-1(ce314) mutant had the similar preference choice to wild-type (data not shown). We observed that nematodes with channelrhodopsin-2 (ChR2)-mediated activation of ADL sensory neurons on assay plates with all-trans retinal (ATR) exhibited significantly enhanced choice index compared with wild-type N2 (Fig. 1c). Preference choice phenotype in nematodes with ChR2-mediated activation of ADL sensory neurons was opposite to that in nematodes with genetically ablated ADL sensory neurons (Fig. 1b,c). The lite-1(ce314) mutant had the similar leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 to wild-type (data not shown). Meanwhile, nematodes with optogenetically activating ADL sensory neurons showed the similar leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 compared with wild-type N2 (Fig. S1). These results suggest the crucial role of ADL sensory neurons in the control of preference choice of nematodes on bacterial food.
Expression of ARR-1 in ADL sensory neurons was required for preference choice control
In C. elegans, arr-1 gene encodes the only G protein-coupled receptor (GPCR) adaptor protein and plays important roles in both longevity and immunity23. We further observed that loss-of-function mutation of arr-1 gene caused the deficit in preference choice behavior compared with wild-type N2 (Fig. S2a). arr-1(ok401) mutant nematodes had similar leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 to those in wild-type N2 (Fig. S2b–S2d). Moreover, we found that expression of arr-1 gene under the control of srh-220 promoter rescued the deficit in preference choice in arr-1(ok401) mutant (Fig. S2a), suggesting that ARR-1 in ADL sensory neurons may be involved in the control of preference choice.
GPCRs in ADL sensory neurons regulated the preference choice behavior
In C. elegans, some genes encoding GPCRs such as SRE-1, SRI-51, SRH-132 and SRH-220 are expressed in ADL sensory neurons24,25,26. Among these genes, we found that loss-of-function mutation of srh-220 gene resulted in enhanced choice index compared with wild-type N2 (Fig. 2a). srh-220(tm3939) mutants had normal leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 (Fig. 2b–d). The deficit in preference choice in srh-220(tm3939) could be rescued by the expression of srh-220 under the control of its own promoter (Fig. 2e).
Roles of SRH-220/GPCR in ADL sensory neurons in the control of preference choice in nematodes.
(a) Effects of srh-220 mutation on preference choice. (b–d) Leaving behavior from bacterial lawns and chemotaxis to OP50 in srh-220 mutants. (e) Rescue assay of preference choice phenotype in srh-220 mutants. (f) Optogenetically activating ADL sensory neurons suppressed the function of SRH-220 in regulating preference choice. Optogenetical activation was performed under the lite-1 mutation background. Control, lite-1(ce314). Bars represent means ± S.E.M. **P < 0.01 vs N2 (if not specifically indicated).
In C. elegans, we further observed that overexpression of srh-220 gene led to the significantly decreased choice index compared with wild-type N2 (Fig. 2f). Moreover, ChR2-mediated activation of ADL sensory neurons inhibited the preference choice phenotype in nematodes overexpressing srh-220 gene (Fig. 2f), implying that activation of ADL may antagonize the function of GPCR/SRH-220 in regulating preference choice in nematodes.
Signaling from ADL sensory neurons in the modulation of preference choice was primarily peptidergic
Previous studies have suggested that signaling from ADL sensory neurons in the modulation of ASH-mediated aversive response is primarily peptidergic27,28. We next examined the role of pathways modulating peptide release from ADL sensory neurons by neuron-specific RNA interference (RNAi). In C. elegans, unc-31 gene encodes a DAG binding protein that plays a key role in dense core vesicle (DCV) release29. ADL RNAi knockdown of unc-31 gene significantly decreased choice index compared with wild-type N2 (Fig. 3a). gsa-1 gene encoded Gas enhances exocytosis from DCVs and pde-4 gene encodes a phosphodiesterase, that is predicted to alter cAMP levels28,29. Similarly, ADL RNAi knockdown of gsa-1 or pde-4 gene significantly decreased choice index compared with wild-type N2 (Fig. 3a). Nematodes with ADL RNAi knockdown of unc-31, gsa-1 or pde-4 gene showed similar leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 to those in wild-type N2 (Fig. 3b–d). These results imply that the signaling from ADL sensory neurons may be primarily peptidergic with respect to the control of preference choice in nematodes. gsa-1(ce94) is a gain-of-function mutant and pde-4(ok1290) is a loss-of-function mutant. We further found that the gsa-1(ce94) mutant had the increased choice index, whereas the pde-4(ok1290) mutant has the decreased choice index compared with wild-type N2 (Fig. S3).
Effects of ADL-specific RNAi of unc-31, gsa-1, or pde-4 gene on preference choice.
(a) Preference choice of nematodes with ADL-specific RNAi of unc-31, gsa-1, or pde-4 gene. (b–d) Leaving behavior from bacterial lawns and chemotaxis to OP50 of nematodes with ADL-specific RNAi of unc-31, gsa-1, or pde-4 gene. Bars represent means ± S.E.M. **P < 0.01 vs N2.
Neuropeptides in ADL sensory neurons were involved in the control of preference choice
In C. elegans, some neuropeptides were expressed in ADL sensory neurons and these neuropeptides are encoded by flp-4, flp-21, nlp-7, nlp-8 and nlp-10 genes30. Among these genes, we found that ADL-specific RNAi knockdown of flp-4 or mutation of nlp-10 significantly decreased choice index compared with wild-type N2 (Fig. 4a). Nematodes with ADL-specific RNAi knockdown of flp-4 and nlp-10(tm6232) mutants had normal leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 (Fig. 4b–d). Therefore, some neuropeptides in ADL sensory neurons may be required for the control of preference choice in nematodes.
Identification of neuropeptides involved in the control of preference choice in nematodes.
(a) Effects of genes encoding neuropeptides expressed in ADL sensory neurons on preference choice. (b–d) Effects of flp-4 or nlp-10 gene on leaving behavior from bacterial lawns and chemotaxis to OP50. (e) Genetic interaction between srh-220 and flp-4 or nlp-10 in regulating preference choice. Bars represent means ± S.E.M. **P < 0.01 vs N2 (if not specifically indicated).
Genetic interaction of SRH-220 with FLP-4 or NLP-10 in regulating preference choice
Moreover, we investigated the genetic interaction between srh-220 and flp-4 or nlp-10 in regulating preference choice in nematodes. We found that the preference choice phenotype in double mutant of flp-4(RNAi);srh-220(tm3783) was similar to that in flp-4(RNAi) nematodes (Fig. 3e). Similarly, the preference choice phenotype in double mutant of nlp-10(tm6232);srh-220(tm3783) was similar to that in nlp-10(tm6232) mutant nematodes (Fig. 3e). Taken together, these results suggest that flp-4 or nlp-10 mutation may suppress the preference choice phenotype in srh-220 mutants in nematodes.
Functional analysis of NPR-4, receptor of FLP-4, in regulating preference choice
In C. elegans, the receptor for the neuropeptide of FLP-4 is NPR-4 and the corresponding receptor for the neuropeptide of NLP-10 is unknown30. Loss-of function mutation of npr-4 gene significantly decreased choice index compared with wild-type N2 (Fig. 5a). Meanwhile, npr-4(tm1782) mutants had normal leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 (Fig. 5b–d). These results suggest that FLP-4, together with its receptor of NPR-4, may be involved in the control of preference choice in nematodes.
NPR-4 regulated the preference choice in nematodes.
(a) Mutation of npr-4 gene altered preference choice. (b–d) Mutation of npr-4 gene did not influence leaving behavior from bacterial lawns and chemotaxis to OP50. (e) Schematic diagram for putative neural networks associated with ADL sensory neurons, extracted from the complete circuit3. Sensory neurons are shown as triangles and interneurons as hexagons. Arrows indicate chemical synapses. (f) Expression of NPR-4 in AIB interneurons rescued the deficit in preference choice in npr-4 mutants. Bars represent means ± S.E.M. **P < 0.01 vs N2 (if not specifically indicated).
In C. elegans, sensory inputs can be released from ADL sensory neurons to AIA, AIB, AVA, AVB, or AVD interneurons3 (Fig. 5e). Among AVA, AVB and AVD interneurons involved in the control of locomotion behavior10, NPR-4 was reported to be able to be expressed in AVA interneurons31. However, expression of NPR-4 in AVA interneurons did not recover deficit in preference choice in npr-4(tm1782) mutants (Fig. 5f). Considering the crucial role of sensory interneurons in the control of behavioral choice6,9, we also examined the function of NPR-4 in AIA or AIB interneurons in regulating preference choice. Interestingly, we found that expression of NPR-4 in AIB interneurons could obviously rescue the deficit in preference choice in npr-4(tm1782) mutants (Fig. 5f). In C. elegans, NPR-4 could be expressed in AIB interneurons (Fig. S4).
Mutation of npr-4 gene altered expression patterns of some genes expressed in AIB interneurons
To identify candidate targeted genes for npr-4 in regulating preference choice, we examined expression patterns of genes expressed in AIB interneurons (http://legacy.wormbase.org/db/searches/expr_search#results). Among 24 genes expressed in AIB interneurons, we found that mutation of npr-4 gene caused the significant increase in expression levels of ptp-3 and ced-10 genes and the significant decrease in expression levels of glr-2, tax-6, cdc-42 and set-2 genes (Fig. 6a). In C. elegans, ptp-3 gene encodes a receptor-like tyrosine phosphatase, ced-10 gene encodes a GTPase, glr-2 gene encodes a glutamate receptor, tax-6 gene encodes a calcineurin A, cdc-42 gene encodes a RHO GTPase and set-2 gene encodes a histone H3 at lysine 4 (H3K4) methyltransferase.
Identification of downstream target for NPR-4 in regulating preference choice in nematodes.
(a) Mutation of npr-4 gene altered expression patterns of some genes expressed in AIB interneurons. (b) Mutation of set-2 gene induced the deficit in preference choice. (c) Genetic interaction of npr-4 with set-2 in regulating preference choice. (d–f) Leaving behavior from bacterial lawns and chemotaxis to OP50 in set-2 mutants. Bars represent means ± S.E.M. **P < 0.01 vs N2.
SET-2 functioned downstream of NPR-4 to regulate preference choice
We further used the available mutants for candidate targeted genes of npr-4 to investigate their possible function in regulating preference choice in nematodes. Among 7 candidate genes examined, mutation of set-2 gene caused the significant decrease in choice index compared with wild-type N2 (Fig. 6b). Moreover, preference choice phenotype of double mutant of set-2(ok952);npr-4(tm1782) was similar to that of single mutant of set-2(ok952) or npr-4(tm1782) (Fig. 6c), suggesting that SET-4 and NPR-4 may function genetically in the same pathway to regulate preference choice in nematodes. set-2(ok952) mutants had similar phenotypes of leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 to those in wild-type N2 (Fig. 6d–f).
Identification of SET-2 mediated signaling pathway in regulating preference choice
In C. elegans, it was reported that ASH, WDR-5 and SET-2 constitute an ASH-2 trithorax complex, which trimethylates histone H3K432. ASH-2 is a trithorax group protein and WDR-5 is a WD40 repeat-containing protein. RBR-2 is a H3K4 demethylase. We found that mutation of ash-2 or wdr-5 gene caused the significant decrease in choice index compared with wild-type N2; however, mutation of rbr-2 did not significantly affect preference choice behavior (Fig. 7a). These results suggest the involvement of ASH-2 trithorax complex in the control of preference choice behavior in nematodes.
SET-2 functioned together with ASH-2 and WDR-5 to regulate preference choice in nematodes.
(a) Preference choice phenotype in ash-2, wdr-5, or rbr-2 mutants. (b) Genetic interaction of SET-2 with ASH-2 or WDR-5 in regulating preference choice. (c) Leaving behavior from bacterial lawns and chemotaxis to OP50 in ash-2 or wdr-5 mutants. (d) The molecular signals regulating preference choice in the neuronal circuit of ADL sensory neuron-AIB interneuron. Sensory neurons are shown as triangles and interneurons as hexagons. Arrows indicate chemical synapses. Bars represent means ± S.E.M. **P < 0.01 vs N2.
In the ASH-2 trithorax complex, genetic interactions further indicate that SET-2 might function in the same pathway with ASH-2 in regulating preference choice (Fig. 7b). Similarly, SET-2 might also function in the same pathway with WDR-5 in regulating preference choice (Fig. 7b). ash-2(tm1726) or wdr-5(ok1417) mutants had similar phenotypes of leaving behavior from OP50 or PA14 lawns and chemotaxis to OP50 to those in wild-type N2 (Fig. 7d,e). Moreover, expression of set-2, ash-2, or wdr-5 in AIB interneurons rescued the deficit in preference choice in corresponding mutant (Fig. S5).
Discussion
Preference choice to food is an important behavior for animals to survival in and adapt to the environment. Preference choice on bacterial food is an important behavioral mechanism for nematodes when they are living in the environment. For the neuronal circuit of preference choice on bacterial food, it has been reported that AWB or AWC sensory neurons are involved in the control of preference choice because of their functions in affecting olfactory perception in nematodes14. ASH and ADL are two important sensory neurons among the amphid neurons and have been proven to have the potentials in sensing and mediating avoidance of noxious environmental stimuli18,19,20. In the assayed preference choice model, two bacterial foods, OP50 and PA14, were used and PA14 is normally considered a noxious environmental stimuli. Using strains with genetically silencing ASH or ADL sensory neurons, interestingly, we found that ASH sensory neurons were not involved in the control of preference choice on bacterial food in nematodes (Fig. 1b). In contrast, we observed that both genetically silencing and ChR2-mediated activation of ADL sensory neurons significantly influenced preference choice on bacterial food (Fig. 1b,c). These results suggest the crucial role of ADL sensory neurons in regulating preference choice on bacterial food in nematodes. In other words, ADL sensory neurons may regulate preference choice to bacterial food through a neuronal regulation mechanism different from AWB or AWC sensory neurons.
ARR-1, the C. elegans beta-arrestin ortholog, is predicted to act as an adaptor protein that potentially activates the GPCRs to regulate different biological processes such as innate immunity and can be used as a tool to examine the possible role of GPCRs in regulating specific phenotype23,33. We further found that mutation of arr-1 gene also noticeably affected preference choice on bacterial food (Fig. S2). In C. elegans, ARR-1 is expressed throughout the nervous system including the ADL sensory neurons33. Moreover, we observed that expression of arr-1 in ADL sensory neurons rescued the deficits in preference choice on bacterial food in arr-1(ok401) mutants (Fig. S2a), implying that GPCRs in ADL sensory neurons may be involved in the control of preference choice on bacterial food in nematodes. Among the GPCRs expressed in ADL sensory neurons, we further identified the GPCR/SRH-220 as a regulator of preference choice to bacterial food. Mutation of srh-220 led to enhanced preference choice index; however, overexpression of srh-220 resulted in decreased preference choice index (Fig. 2).
Previous studies have suggested that the peptide signaling may play a prominent role in the control of behavioral choice and concentrations of peptides may encode the internal states influencing behavior choice of nematodes6. The signaling from ADL sensory neurons may be primarily peptidergic with respect to the control of some behaviors such as modulation of ASH-mediated aversive response27,28. By analyzing the phenotype in nematodes with ADL RNAi knockdown of unc-31, gas-1, or pde-4 gene, we found that ADL RNAi knockdown of unc-31, gas-1, or pde-4 gene resulted in decreased preference choice index (Fig. 3), suggesting that ADL sensory neurons may also regulate preference choice behavior through the peptidergic signals in nematodes. Among the genes encoding neuropeptides expressed in ADL sensory neurons, we found that two genes, flp-4 and nlp-10, were involved in the control of preference choice behavior (Fig. 4a–d), implying that the neuropeptides encoded by these genes in ADL sensory neurons may be required for the control of preference choice.
In ADL sensory neurons, we found that mutation of flp-4 or nlp-10 gene suppressed preference choice phenotype caused by srh-220 gene mutation (Fig. 4e), suggesting that GPCR/SRH-220 may regulate the preference choice through influencing the function of FLP-4 or NLP-10 in nematodes. More importantly, we found that activation of ADL sensory neurons suppressed the preference choice behavior in nematodes overexpressing srh-220 gene (Fig. 2f), suggesting that ADL activity may regulate preference choice to bacterial food by inhibiting function of GPCR/SRH-220. Therefore, our results raise a signal cascade of GPCR/SRH-220-FLP-4/NLP-10 in ADL sensory neurons involved in the control of preference choice behavior. That is, activation of ADL sensory may regulate preference choice behavior by increasing function of FLP-4 or NLP-10 through the inhibition of GPCR/SRH-220 in nematodes.
Previous studies have implied three main neuronal circuit motifs for the control of behavioral choice in nematodes6. These motifs involve the changes in the strength of synaptic connections or changes in the basal activity of interneutons or sensory neurons to which interneurons are electrically coupled6. In C. elegans, synaptic connections can be established between ADL sensory neurons and AIA, AIB, AVA, AVB, or AVD interneurons3 (Fig. 5e). Considering that mutation of npr-4 gene encoding candidate receptor for flp-4 resulted in the deficit in preference choice behavior (Fig. 5a–d), we investigated the neuron-specific activity of NPR-4 in regulating preference choice behavior. Interestingly, we found that expression of npr-4 gene in AIB interneurons rescued the deficit in preference choice in npr-4 mutants (Fig. 5f), suggesting that the released FLP-4 from ADL sensory neurons may regulate preference choice behavior through the functions of its receptor of NPR-4 in AIB interneurons.
For the underlying molecular mechanism of NPR-4 in regulating preference choice in AIB interneurons, we found that NPR-4 in AIB interneurons may regulate preference choice through the function of SET-2, a histone H3K4 methyltransferase. Several lines of evidence were raised to support this notion. Firstly, expression of set-2 gene was altered by npr-4 mutation (Fig. 6a). Secondly, mutation of set-2 gene led to the decrease in choice index (Fig. 6b). Thirdly, genetic assay suggests that SET-4 and NPR-4 may function in the same pathway to regulate preference choice (Fig. 6c).
For the SET-2 mediated signaling pathway in regulating preference choice, we found that SET-2 acted in the same pathway with the other two members in the ASH-2 trithorax complex, ASH-2 and WDR-5, to regulate preference choice behavior (Fig. 7a,b). These results imply the possible pivotal role of trimethylation of histone H3K4 in the control of preference choice in nematodes. Interestingly, we did not detect the obvious involvement of RBR-2, a H3K4 demethylase, in the control of preference choice behavior (Fig. 7a). Therefore, in the neuronal circuit of “ADL sensory neuron-AIB interneuron”, the released neuropeptide of FLP-4 may regulate preference choice by activating its receptor of NPR-4 in AIB interneurons (Fig. 7f). NPR-4 may further regulate preference choice through the functions of an ASH-2 trithorax complex.
In summary, our data show that ADL sensory neurons were involved in the control of preference choice behavior. Function of ARR-1 in ADL sensory neurons suggests the potential function of GPCRs in ADL sensory neurons in the control of preference choice. In ADL sensory neurons, the GPCR/SRH-220 was identified to regulate preference choice by affecting the function of neuropeptide of FLP-4 or NLP-10. Based on the functional analysis of FLP-4 and its receptor of NPR-4, we raised a neuronal circuit of “ADL sensory neuron-AIB interneuron” involved in the control of preference choice. NPR-4 further regulated preference choice through the functions of an ASH-2 trithorax complex consisting of SET-2, ASH-2 and WDR-5, implying that NPR-4 may regulate preference choice behavior by mediating a molecular machinery for the trimethylation of histone H3K4 in nematodes.
Methods
C. elegans and bacterial strains and genetics
Nematodes were grown on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C as described34. The following strains were used in the current study: wild-type Bristol N2, mutants of lite-1(ce314) X, arr-1(ok401) X, srh-220(tm3939) IV, flp-21(ok889) V, nlp-7(tm1970) X, nlp-8(ok1799) I, nlp-10(tm6232) III, npr-4(tm1782) X, tax-6(ok2065) IV, glr-2(ok2342) III, ced-10(n1993) IV, ptp-3(ok244) II, cdc-42(ok825) II, gsa-1(ce94) I, pde-4(ok1290) II, set-2(ok952) III, ash-2(tm1726) II, wdr-5.1(ok1417) III and rbr-2(ok2544) IV and transgenic strains of quEx128[npr-9::GFP + pRF4 rol-6(su1006)] expressing npr-9 in AIB interneurons and ASH::TeTx20. All the used mutants were backcrossed to N2 for at least five times. Double mutant strains without additional marker mutations were constructed using standard genetic methods and verified by complementation testing. At least five independent lines were examined for each rescue experiment.
Bacterial strains used were E. coli OP50 and P. aeruginosa PA14. E. coli and P. aeruginosa cultures were grown in Luria-Bertani (LB) broth overnight at 37 °C.
Behavioral assay
Behavioral choice was assayed as described previously12,14. Embryos collected by bleaching were grown on a standard NGM plate fed with E. coli OP50. All the behavioral experiments were performed with young adults maintained at 20 °C. Behavioral choice assay was performed on 9-cm NGM plates. Bacterial food with a diameter of 0.5-cm was seeded at the distance of 1.5 cm from periphery (Fig. 1a). Animals were then put on the center of the assay plates and allowed to migrate. Number of nematodes on each bacterial lawn was counted after treatment for 2 h at 20 °C. Behavioral choice index was calculated as (PA14 number – OP50 number)/total number. In this behavioral choice assay system, choice index of −1.0 represents the complete preference for OP50, choice index of 1.0 represents the complete preference for PA14 and choice index of 0 represents an equal distribution. Ten replicates were performed.
The method for assay of leaving behavior from OP50 or PA14 lawn was performed as described35. Small lawns of PA14 or OP50 were cultured on 6-cm NGM plates overnight at 25 °C and 20 young adults were put in the center of each bacteria lawn. The number of animals on each lawn was counted after 16 h. Ten replicates were performed.
To analyze the chemotaxis perception of nematodes to OP50, OP50 with a diameter of 0.5-cm was seeded at one side with the distance of 1.5 cm from periphery on 9-cm NGM plates. M9 buffer was added to the other side with the distance of 1.5 cm from periphery on 9-cm NGM plates. Nematodes were put on the center of the assay plates and allowed to migrate for 2 h. The chemotaxis index was calculated as (OP50 number – M9 buffer number)/total number. Ten replicates were performed.
DNA constructs and germline transformation
To generate entry vectors carrying promoter sequences, promoter regions were amplified by polymerase chain reaction (PCR) from wild-type C. elegans genomic DNA (1.6 kb for srh-220 promoter used for ADL-specific expression26, 2.8 kb for gcy-28.d promoter used for AIA-specific expression, 1.9 kb for npr-9 promoter used for AIB-specific expression and 1.8 kb for pept-3 promoter used for AVA-specific expression and then inserted into the pPD95_77 vector in the sense orientation. egl-1, arr-1, srh-220, npr-4, set-2, ash-2, or wdr-5 cDNA was amplified by PCR. The sequences of the amplified cDNA were verified by sequencing and then cDNA was inserted into corresponding entry vectors carrying promoter sequence. To construct Psrh-220-ChR2 and Pnpr-9-ChR2 for optical activation of ADL sensory neurons or AIB interneurons, the srh-220 or npr-9 promoter fragment was inserted into the 95_75-ChR2 vector. To determine whether npr-4 is expressed in AIB interneurons, the npr-4 promoter (2.7 kb) was inserted into 95_77-mcherry vector and then transformed into the strain of quEx128. Germline transformation was performed as described36 by coinjecting the testing DNA at a concentration of 10–40 μg/mL and the marker DNA of Pdop-1::rfp or Plin-44::gfp at a concentration of 60 μg/mL into the gonad of nematodes.
Optical genetic assay
Light-activated ChR2, a directly light-gated cation channel from the green alga Chlamydomonas reinhardtii, can utilize chromophore ATR to enable the activity of specific neurons in C. elegans37. The srh-220 promoter was used for the ADL-specific expression and the npr-9 promoter was used for the AIB-specific expression. Nematodes expressing ChR2 in ADL sensory neurons or AIB interneurons were grown on OP50-seeded NGM agar plates containing 50 μM of ATR. Plates were seeded on day 0. Nematodes were transferred to OP50-seeded plates containing 50 μM of ATR on day 1 in the dark. After starvation for 5 h, nematodes were transferred to assay plates containing 50 μM of ATR for behavioral analysis on day 2. During the assay, whole field illumination was performed and ChR2 was excited by a round blue light in diameter of 9.5 cm sourced from LED array (460–470 nm, ~0.5 mW/mm2) constructed in a LED light source (Chenyufanli Trading Co. LTD., Nanjing, China). Light intensity measured at the sample was 5 mW/mm2 of 465 nm light. For optogenetic experiments, light intensity was monitored using an optical power meter (PM100, Thorlabs). All work was carried out under low-illumination conditions to prevent the preactivation of ChR2-expressing neurons. Nematodes without ATR treatment were used as the control. Optogenetical activation was performed under the lite-1 mutation background. Ten replicates were performed.
RNAi
RNAi was performed by feeding nematodes with E. coli strain HT115 (DE3) expressing double-stranded RNA that is homologous to a target gene as described38. E. coli HT115 (DE3) grown in LB broth containing ampicillin (100 μg/ml) at 37 °C overnight was plated onto NGM containing 100 μg/mL ampicillin and 5 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG). L2 larvae were placed on RNAi or vector control plates for 2 days at 20 °C until nematodes became gravid. Gravid adults were transferred to fresh RNAi-expressing bacterial lawns and allowed to lay eggs for 2 h to obtain the second generation of RNAi population. Eggs were allowed to develop at 20 °C to young adults for the subsequent assays. unc-22 RNAi was included as a positive control.
ADL neuron-specific RNAi
The method was performed basically as described39. The exon rich fragment of genomic sequence of flp-4, pde-4, unc-31, or gsa-1 gene was amplified by PCR to yield product A. The srh-220 promoter was used for ADL-specific expression and amplified in two different reactions with promoter reverse sense primer or promoter reverse antisense primer together with promoter forward primer to yield product B and C. Product A was further fused by amplification to B or C using the nested primers to yield fragments D and E. In fragment D, the flp-4, pde-4, unc-31, or gsa-1 gene was transcribed by ADL-specific promoter in the sense orientation. In fragment E, the flp-4, pde-4, unc-31, or gsa-1 gene was transcribed by the ADL-specific promoter in the antisense orientation. The fragments D and E were mixed in equimolar amounts and injected at 25–100 ng/μL into the wild-type nematodes. The Plin-44::gfp was used as a transgenic marker.
Reverse transcription and quantitative real-time PCR (qRT-PCR)
Total RNA of nematodes was extracted using RNeasy Mini Kit (Qiagen, Valencia, Ca, USA). Purity and concentration of RNA were evaluated by OD260/280 in a spectrophotometer. Total RNA was reverse-transcribed using cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). After cDNA synthesis, real-time PCR was performed using SYBR Premix Ex Taq™ (Takara) for amplification of the PCR products. The act-1 gene was chosen as a reference gene. Relative quantification of targeted genes in comparison to reference act-1 gene was determined and the final results were expressed as relative expression ratio between targeted gene and reference gene. The primer information was shown in Table S1. Three replicates were performed.
Statistical analysis
All data in this article were expressed as means ± standard error of the mean (S.E.M.). Graphs were generated using Microsoft Excel (Microsoft Corp., Redmond, WA). Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, USA). Differences between groups were determined using analysis of variance (ANOVA). The probability levels of 0.05 and 0.01 were considered to be statistically significant.
Additional Information
How to cite this article: Yu, Y. et al. FLP-4 neuropeptide and its receptor in a neuronal circuit regulate preference choice through functions of ASH-2 trithorax complex in Caenorhabditis elegans. Sci. Rep. 6, 21485; doi: 10.1038/srep21485 (2016).
References
Rangel, A., Camerer, C. & Montague, P. R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 9, 545–556 (2008).
Palmer, C. R. & Kristan, W. B. Jr. Contextual modulation of behavioral choice. Curr. Opin. Neurobiol. 21, 520–526 (2011).
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986).
Bargmann, C. I. Chemosensation In C. elegans. Wormbook. 1–29 (2006).
Ye, H.–Y., Ye, B.–P. & Wang, D.-Y. Learning and learning choice in the nematode Caenorhabditis elegans. Neurosci. Bull. 22, 355–360 (2006).
Faumout, S., Lindsay, T. H. & Lockery, S. R. Neuronal microcircuits for decision making In C. elegans. Curr. Opin. Neurobiol. 22, 580–591 (2012).
Li, Y.–X., Wang, Y., Hu, Y.–O., Zhong, J.–X. & Wang, D.-Y. Modulation of the assay system for the sensory integration of 2 sensory stimuli that inhibit each other in nematode Caenorhabditis elegans. Neurosci. Bull. 27, 69–82 (2011).
Wang, D.–Y., Yu, Y.–L., Li, Y.–X., Wang, Y. & Wang, D.-Y. Dopamine receptors antagonistically regulate behavioral choice between conflicting alternatives In C. elegans. PLoS ONE 9, e115985 (2014).
Ishihara, T. et al. HEN-1, a secretary protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109, 639–649 (2002).
Kawano, T. et al. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron 72, 572–586 (2011).
Matsuura, T., Oikawa, T., Wakabayashi, T. & Shingai, R. Effect of simultaneous presentation of multiple attractants on chemotactic responses of the nematode Caenorhabditis elegans. Neurosci. Res. 48, 419–429 (2004).
Zhang, Y., Lu, H. & Bargmann, C. I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438, 179–184 (2005).
Bargmann, C. I., Hartwieg, E. & Horvitz, H. R. Odorant-selective genes and neurons mediate olfaction In C. elegans. Cell 74, 515–527 (1993).
Abada, E. A. et al. C. elegans behavior of preference choice on bacterial food. Mol. Cells 28, 209–213 (2009).
Meisel, J. & Kim, D. H. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 35, 465–470 (2014).
Ha, H. I. et al. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68, 1173–1186 (2010).
Harris, G. et al. Dissecting the signaling mechanism underlying recognition and preference of food odors. J. Neurosci. 34, 9389–9403 (2014).
Bargmann, C. I., Thomas, J. H. & Horvitz, H. R. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 55, 529–538 (1990).
Sambongi, Y. et al. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport 11, 2229–2232 (2000).
Guo, M. et al. Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nat. Commun. 6, 5655 (2015).
Styer, K. L. et al. Innate immunityy in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322, 460–464 (2008).
Schmitt, C., Schultheis, C., Husson, S. J., Liewald, J. F. & Gottschalk, A. Specific expression of channelrhodopsin-2 in single neurons of Caenorhabditis elegans. PLoS ONE 7, e43164 (2012).
Singh, V. & Aballay, A. Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by Arrestin-1. J. Biol. Chem. 287, 33191–33197 (2012).
Troemel, E. R., Chou, J. H., Dwyer, N. D., Colbert, H. A. & Bargmann, C. I. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218 (1995).
McCarroll, S. A., Li, H. & Bargmann, C. I. Identification of transcriptional regulatory elements in chemosensory receptor genes by probabilistic segmentation. Curr. Biol. 15, 347–352 (2005).
Mohan, S., Timbers, T. A., Kennedy, J., Blacque, O. E. & Leroux, M. R. Striated rootlet and nonfilamentous forms of rootletin maintain ciliary function. Curr. Biol. 23, 2016–2022 (2013).
Harris, G. et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J. Neurosci. 30, 7889–7899 (2010).
Mills, H. et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J. 31, 667–678 (2012).
Zhou, K. M. et al. PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56, 657–669 (2007).
Li, C. & Kim, K. Neuropeptides, WormBook, ed. The C. elegans Research Community, WormBook, doi: 10.1895/wormbook.1.142.1 (2008).
Cohen, M. et al. Coordinated regulation of foraging and metabolism in C. elegans by RFamide neuropeptide signaling. Cell Metab. 9, 375–385 (2009).
Greer, E. L. et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 466, 383–387 (2010).
Palmitessa, A. et al. Caenorhabditis elegans arrestin regulate neural G protein signaling and olfactory adaptation and recovery. J. Biol. Chem. 280, 24649–24662 (2005).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Pradel, E. et al. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 2295–2300 (2007).
Mello, C. & Fire, A. DNA transformation. Methods Cell. Biol. 48, 451–482 (1995).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Kamath, R. K., Martinez-Campos, M., Zipperlen, P., Fraser, A. G. & Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double stranded RNA in C. elegans. Genome Biol. 2, 1–10 (2001).
Esposito, G., Di Schiavi, E., Bergamasco, C. & Bazzicalupo, P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395, 170–176 (2007).
Acknowledgements
Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Dr. Di Chen for providing E. coli HT115 strains expressing double-stranded RNA for the examined genes and Dr. Zhengxing Wu for providing the strain of ASH::TeTx. This work was supported by the grants from National Natural Science Foundation of China (81172698).
Author information
Authors and Affiliations
Contributions
Y.Y., L.Z., X.G. and D.Y.W. (Daoyong Wang) performed the experiments. D.Y.W. (Dayong Wang) conceived the experiments, interpreted the data and wrote the manuscript with help from all of the other authors. D.Y.W. (Dayong Wang) supervised the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, Y., Zhi, L., Guan, X. et al. FLP-4 neuropeptide and its receptor in a neuronal circuit regulate preference choice through functions of ASH-2 trithorax complex in Caenorhabditis elegans. Sci Rep 6, 21485 (2016). https://doi.org/10.1038/srep21485
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21485
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.