Abstract
Study design: Cross sectional comparison, control group.
Objective: To determine if incomplete spinal cord injured patients (SCI) have an abnormal blood flow response to cuff ischemia compared to able-bodied individuals (AB).
Setting: Academic institution.
Methods: Blood flow in five chronic incomplete SCI patients (C4–C5) and 17 able bodied individuals was measured in the common femoral artery using quantitative Doppler ultrasound (GE LogiQ 400CL) at rest and after distal thigh cuff occlusion of 2, 4 and 10 min to investigate whether blood flow or vascular control were different in SCI's and AB.
Results: Blood flow and the diameter of the common femoral artery at rest were similar in incomplete SCI and AB. Peak flow after 10 min of cuff ischemia (the highest found) was also comparable between incomplete SCI and AB. The half-time for recovery of blood flow to baseline after 2, 4 or 10 min of ischemia was 50% longer for incomplete SCI compared to the AB (P=0.023). In addition, peak blood flow after 2 and 4 min of ischemia relative to the maximum, 10 min value (2/10 and 4/10 ratios) was lower in incomplete SCI compared to AB (0.65±0.06 vs 0.76±0.15, P=0.029 and 0.75±0.10 vs 0.89±0.11, P=0.014, respectively).
Conclusion: This study demonstrated that incomplete spinal cord injured patients have impaired vascular control seen as a slower return to resting flow after cuff ischemia and reduced sensitivity to ischemia relative to maximum flow. However, incomplete SCI patients did not demonstrate impaired flow capacity as seen in complete SCI patients suggesting that smaller cardiovascular abnormalities are seen with incomplete versus complete SCI injury. Impaired vascular control may serve to limit exercise capacity and may contribute to increased cardiovascular disease. Impaired circulation could contribute to impaired muscle function and poor cardiovascular health in incomplete SCI's, although these findings need to be replicated in a study with more subjects.
Sponsorship: Paralyzed Vetrans Association and NIH grants HL65179 and HD33738.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) results in profound changes within the affected limbs. Within several months of injury, skeletal muscle mass and muscle fiber size can be a half to one third the size of that of able-bodied subjects (AB).1,2 In addition, several studies have reported chronic SCI patients to have femoral artery diameters and maximal blood flow with 5 min of cuff ischemia that are 50% of AB individuals.3,4 These peripheral circulatory and skeletal muscle adaptations may contribute to the increased risk of cardiovascular disease in SCI patients.5 Kocina et al6 have indicated that electrical stimulation has the potential to improve overall cardiovascular fitness. However, muscles in people with SCI have been shown to be more fatiguable than those in AB controls,1,7 possibly due to their impaired circulation.8 Thus, the increased fatigability of SCI muscle might be an impediment to using electrical stimulation to improve cardiovascular fitness.
As many as 55% of SCI patients have incomplete injury, in that they retain some (reduced) motor and/or sensory function in the affected limbs.9 Incomplete injuries are associated with quicker stabilization after injury and a greater extent of recovery than complete injuries.9,10,11 Previous studies have measured arterial blood flow in SCI patients with complete injuries.3,4 Very little research has been done investigating individuals with incomplete injuries. One might predict that incomplete SCI individuals would show similar deficits in blood flow as complete SCI patients, although perhaps less in magnitude.
Reactive hyperemia (cuff ischemia) is one of the best methods to study hemodynamics in SCI patients for several reasons.3,4 First, resting metabolic demands are so low that resting blood flow might be of little diagnostic value. Second, blood flow during exercise is influenced by cardiac output, making changes in peripheral vascular function hard to separate out. Finally, incomplete SCI patients could have difficulty exercising at adequate intensities to stimulate maximal blood flow. Reactive hyperemia has been extensively used as a research tool. Sensitivity to reactive hyperemia has received less attention, however, even though it provides additional insight into vascular function. In light of the aforementioned, the purposes of this study were to determine if incomplete SCI patients had reduced peak blood flow and reduced arterial diameters compared to AB individuals. In addition, we tested the hypothesis that SCI results in reduced sensitivity to reactive hyperemia. Lastly, we wanted to determine if there are differences in cuff duration that are needed to elicit a peak reactive hyperemic response in SCI's or AB.
Methods
Subjects
Five patients with incomplete chronic SCI and 17 AB subjects volunteered to participate in the study. The AB group was recruited from the university community while the SCI's were recruited from the Shepherd Center in Atlanta, Georgia. SCI patient's injuries were at the C4–C5 level and all had been injured for 1 to 3 years (Table 1). The SCI patients had not been injured for an extended time and thus, would be expected to have few long-term complications. All SCI individuals had some sensory and motor control in the affected limbs. Four of the five were ambulatory (one with a walker) while the remaining subject had moderate movement but relied on a wheelchair. None of the subjects reported regular physical activity. The SCI group was mobile and thus, was more active than complete SCI injured individuals that have been tested in previous literature, however, they were less mobile than the control group. All subjects were tested after a low fat meal and none had any history of disease or other confounding factors. The study was conducted with the approval of the Institutional Review Board at the University of Georgia.
Protocol
Subjects were asked to abstain from fatty foods for at least 6 h prior to testing. This was done to eliminate the potential for diet to confound the results.12 Height, weight and age were recorded. Subsequently, subjects rested in a supine position for 10–15 min prior to testing. The experimental arrangement is shown in Figure 1. Blood pressure was measured in the arm using an automated blood pressure machine (DatascopeA). Resting diameter and blood flow were measured in the common femoral artery using Doppler ultrasound. Three trials of leg ischemia were performed by inflating a cuff about the distal thigh. There was 5 min of recovery between trials. The durations of cuff ischemia were 2, 4 and 10 min. Preliminary studies suggest that there is no order effect for duration of cuff ischemia. Thus, longer durations of ischemia were done last to minimize discomfort to the subject. Ischemia was induced in the distal thigh and leg by inflation of a blood pressure cuff distal to the probe to a pressure 100 mmHg above systolic. Cuff inflation and deflation were rapid (1–2 s) and performed using a Hokanson device. In addition, continuous recordings of blood pressure were used to ascertain if blood pressure changes occurred during the test as a result of cuff ischemia (Finapres, OhmedaB). Following ischemic tests, leg volume was determined.
Blood flow
Blood flow was measured in the common femoral artery using quantitative Doppler ultrasound (GE LogiQ 400CLC). A linear array transducer was used at a frequency of 6 MHz. The imaging site was located on the upper third of the thigh and was marked to ensure replication of probe placement. Doppler measurements were made proximal to the cuff to ensure that the vessel placement was maintained throughout cuff occlusion. Resting diameter was measured in the axial view during diastole. Pulsed Doppler ultrasound was recorded in the longitudinal view using an insonation angle of 60°. The velocity gate was set to include the entire arterial diameter. Measurements were made continuously and averaged over two heartbeats. All data were saved to magnetic optical disks for storage and analysis.
Doppler waveforms were analyzed to determine maximum (Vmax) and minimum (Vmin) velocity, the time average maximum velocity (Tmax), and time average mean velocity (Tmean). All calculations were done by GE's advanced vascular program software for the LogiQ 400CL. Waveforms that were not automatically measured by the computer were manually traced to determine velocities. B mode images were marked and measured to determine the diameter throughout the test (DIA). Peak systolic blood flow (Max BF) was calculated by the product of vessel cross sectional area and Tmax. Mean systolic blood flow (Mean BF) was calculated by the product of vessel cross sectional area and Tmean. Blood flow values were saved and recorded every 5–10 s during the first minute and then every 15–20 s during the last 4 min of recovery. A total of 20–30 measurements were made for each cuff duration.
Maximum blood flow was determined as the highest Max BF for each ischemic test. As the 10 min cuff duration always had the largest Max BF values, this value was used to compare maximum blood flow between SCI and AB groups. The half time to recovery was determined as the time where blood flow dropped to one half the magnitude between maximum flow and resting flow.
Vascular control
Vascular control was evaluated by two different measurements. First, the halftime for recovery of peak flow to baseline after 2, 4 and 10 min of ischemia was used to indicate the ability of the arteries to resume resting vascular tone. Second, reactivity was assessed as the peak blood flow response to 2 and to 4 min of cuff ischemia relative to the peak blood flow response to 10 min of ischemia (2/10 and 4/10).
Leg volume
Leg volumes were calculated from measurements of fat thickness by Doppler ultrasound and by circumference measurements of the leg. Doppler images of the thickness between skin to muscle fascia were attained every 3 cm over the medial gastrocnemius and over the anterior tibialis to determine the amount of subcutaneous fat. Total area of the leg was determined from the circumference measured and fat thickness. Based on this information fat volume, lean volume, and total volume was calculated.
Analysis
Independent samples t-tests (SPSS version 9.0) were conducted on all data to compare differences between the two groups. The data were analyzed to verify normality and to test for any outliers. Levene's test was conducted to determine equality of variances and was corrected if inequality was found. All analyses were conducted at a significance level of 0.05. Repeated measures ANOVA's were calculated to determine if maximal blood flow or half time to recovery was significantly different between the AB and SCI groups across all time intervals.
To our knowledge no research has looked at vascular function in individuals with incomplete SCI injury, thus, it is difficult to determine the sample size needed to determine adequate power. However, based on prior literature with individuals with complete SCI injury large decreases have been found in blood flow and diameter size.13 Based on this literature a sample size of five individuals would give a power of 0.80 at a significance level of α=0.05.
Results
Resting
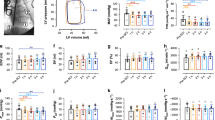
No significant differences were found between the AB group and the SCI group for age, height, weight, total leg volume, lean leg mass, or fat leg mass (Table 2). SCI patients resting blood flow per 100 g of lean tissue (normalized flow) was 53% greater than AB (t(17)=−2.326, P=0.033, d=1.09) (Table 3). No other significant differences were found between groups at rest.
Post cuff ischemia
All subjects were able to tolerate cuff occlusion. There were no significant differences between SCI and AB groups in heart rate or blood pressure during or immediately after any of the cuff occlusions. During cuff ischemia blood flow was reduced to ∼25% of resting flow instead of zero because of the proximal placement of the Doppler probe relative to the cuff. Upon release of the cuff, there was an initial burst of blood flow with the first heartbeat and then a large hyperemic response. Ignoring the first heart beat, the flow response increased to a peak value 4–17 s after release of the cuff and then returned to resting values (Figure 2). The peak flow response was dependent upon duration of ischemia (10 min>4 min>2 min, F(2,36)=88.866, P<0.001, ζ2=0.117). There was no significant difference in time to peak flow response between groups across all time periods. Max BF was not significantly different between SCI and AB groups for all ischemic durations. When blood flow was normalized to lean leg volume, there was no difference between the SCI and AB groups (Figure 3).
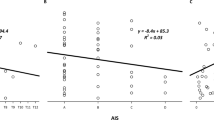
We found that the time to return to resting flow also increased with increasing duration of ischemia for each group (10 min>4 min>2 min). The time it took for flow to decline from peak to resting for any cuff occlusion was ∼50% longer in the SCI patients compared to AB controls F(1,18)=6.131, P=0.023, ζ2=0.254 (Table 4 and Figure 4). The sensitivity of vasodilatation, as measured by the ratio of peak flow after 2 and 4 min relative to 10 min of ischemia (2/10 and 4/10, respectively), was significantly lower in SCI patients (t(16.9)=2.389, P=0.029, d=0.99 and t(20)= 2.680, P=0.014, d=1.41 respectively, see Figure 5 ).
Discussion
The primary finding of this study was that incomplete SCI patients had evidence of abnormal vascular control. This was indicated by prolonged recovery of flow after ischemia and reduced flow sensitivity to ischemia via ratios of peak flow. Abnormal vascular control has not been reported previously in SCI patients. However, abnormal vascular control has been seen in a variety of conditions including: aging,14,15 race,16,17 hypercholesterolemia,18,19 obesity,20 diabetes21,22,23,24 and examples of muscle deconditioning after 14–20 days of bed rest.24,25,26,27 Furthermore, untrained individuals have been shown to have poor orthostatic tolerance when compared to trained individuals.25 Spinal cord injury provides an extreme case of disuse reflected by the abrupt, marked atrophy of the affected muscles1 and ultimately a predominant shift towards type 2 fibers.26 Decreased physiological response in relation to inactivity has been seen in patients that have been immobilized and has been related to muscle atrophy or a decrease in capillarization.27 Thus, decreased activity of incomplete SCI's may be one possible explanation for the impaired vascular function found in this study.
Physical activity was not quantified in this study. However, it is likely that the SCI group was still inactive relative to our controls. The SCI's verbally reported less physical activity than the AB group. Furthermore, two of the members of the SCI group were reliant on a chair or walker and the others reported some sort of assistance needed in some activities of daily life. Reduced activity levels in the SCI's were not consistent with the lack of change in leg volume or composition as seen in this study. However, physiological changes in circulation do not require significant reductions in muscle composition. Perhaps more sensitive measurements of muscle mass, such as magnetic resonance imaging techniques, may have detected changes in leg volume or composition in incomplete SCI's.
Other explanations have been provided as to why vascular function may be altered. Kamiya et al28 believed that vasodilation was attenuated because of increases in muscle sympathetic nervous activity, impairment of β-adrenergic vasodilation, and impairment of nitric oxide release. Still others have indicated that the interruption of neurological impulses causes metabolic changes in blood vessels which result in altered venous competence.29 Lastly, another possible explanation could be a decrease in nitric oxide release from the endothelium due to inactivity,30 or disease status.23 A decrease in nitric oxide or in the number of receptors for nitric oxide would result in a diminished sensitivity to cuff occlusion. A possible hypothesis to explain our findings is that incomplete SCI individuals have reduced sympathetic tone. This hypothesis is supported by the findings of Houtman et al31,32 in which sympathetic nervous system response was altered in SCI's compared to AB. Still others have found that resting sympathetic tones is unaltered in quadriplegics but it was postulated to be dampened or altered under some conditions.33
Two different approaches were used to assess altered vascular control in this study, halftime to recovery and the ratios of peak flow. The half time to recovery has been used as an index of activity related changes in vascular control. Previous studies have shown slower half time to recovery in patients who were immobilized,34 detrained,27 and in patients with vascular disease.35 We also used a novel method of assessing vascular control, the ratios of peak flow (2/10 and 4/10). This approach was modeled after in vitro studies and in situ pharmacological studies that assess flow responses to various infused concentrations of vasoactive substances.36 Deconditioning has been shown to cause an attenuation in flow increase with acetylcholine infusion.37 Our results are consistent with these findings of decreased sensitivity to vasodilating substances, and did not require invasive procedures. The various cuff durations also provided us with information regarding SCI response to reactive hyperemia. Our results indicated that peak reactive hyperemia in incomplete SCI individuals can be obtained with 10 min of cuff duration and that the response is similar to those found in AB individuals.
Interestingly, we did not find evidence of reduced flow capacity in incomplete SCI patients. Previous studies on complete SCI patients reported large (50%) decreases in femoral artery diameter and flow capacity.3,4 Our findings indicate that blood flow when normalized to muscle mass and femoral artery diameter was 8% lower in the SCI's but not statistically significant. These findings could possibly be explained by a low sample size but even if the sample size were increased one would not expect to find as large changes in the incomplete SCI patients compared to the previous literature of complete SCI's. Smaller changes in the incomplete SCI patients may reflect the lesser degree of inactivity in these patients compared to the complete tetraplegics and paraplegics studied by Nash and Hopman, respectively3,4 or a shorter duration of injury. Flow capacity is important for exercise capacity; however, it is not the only determinant of cardiovascular function. Vascular control has also been shown to be impaired in black subjects compared to white subjects, even though both groups had similar flow capacity.36 Impaired flow control despite similar flow capacity was suggested as a cause of insulin insensitivity, hypertension, and increased risk of cardiovascular disease.5 More research investigating vascular control and atrophy in incomplete SCI's is needed, as many of the injuries reported are incomplete injuries.
One of the limitations to this study was the small number of incomplete SCI patients. Had the effect size been as large as the effect size seen in previous complete SCI studies (femoral artery diameters 50% less than control),13 the sample size used in this study would have had adequate power (β<0.80). In addition, the small effect sizes of blood flow capacity and diameters may not be of functional significance. More likely to be of functional significance would be the variables that we did find statistically significant, such as the 50% decrease in half time to recovery.
There are several consequences of abnormal vascular control in incomplete SCI patients. The first is that it might contribute to insulin insensitivity and hypertension.5 The second is that it might decrease oxygen delivery and contribute to muscle fatigue. Muscle fatigue is multifactoral,8 but impaired oxygen delivery has been shown to contribute to increased rates of fatigue.38 Consistent with this suggestion are studies that have suggested that long term SCI patients have increased fatiging that is not explained by reduced oxidative enzymes.39,40
In summary, this study is one of the first to examine incomplete SCI, who make up as much as 55% of all SCI patients. These findings suggest that incomplete spinal cord injured patients have impaired vascular control. However, incomplete SCI patients did not demonstrate impaired flow capacity as seen in complete SCI patients suggesting that smaller cardiovascular abnormalities are seen with incomplete versus complete SCI injury. Impaired vascular control may serve to limit exercise capacity and may contribute to increased cardiovascular disease. Lastly, this study provided evidence that 10 min of cuff occlusion produces similar maximal reactive hyperemia effects in incomplete SCI and AB individuals. Future studies will be needed to confirm these findings, as well as to test if impaired vascular control is important in the function and health of these patients. If these results were confirmed, improving peripheral arterial function would be an important part of any treatment program for SCI patients.
References
Castro M et al. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury Eur J Appl Physiol 1999 86: 350–358
Castro M, Apple D, Hillegass A, Dudley G . Influence of complete spinal cord injury on skeletal muscle cross-sectional area with six months of injury Eur J Appl Physiol 1999 80: 373–378
Hopman MTE et al. Properties of the venous vascular system in the lower extremities of individuals with paraplegia Paraplegia 1994 32: 810–816
Nash MS, Montalvo BM, Applegate B . Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons Arch Phys Med Rehabil 1996 77: 1260–1265
Villa E, Gonzalez-Albarran O, Rabano A, Garcia-Robles R . Effects of hyperinsulinemia on vascular blood flows in experimental obesity Ster Biochem Molec Biol 1999 69: 273–279
Kocina P . Body composition of spinal cord injured adults Intl J Sports Med 1997 19: 98–103
Shields R . Fatiguability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle J Neurophysiol 1995 73: 2195–2206
Edwards RHT . Biochemical bases of fatigue in exercise performance: catastrophe theory of muscular fatigue. In: Knuttgen HG, Vogel JA, Portmans JR (eds) Biochemistry of Exercise Vol 13: Champagne, IL: Human Kinetics Publisher 1983 pp 3–28
Sekhon L, Fehlings M . Epidemiology, demographics and pathophysiology of acute spinal cord injury Spine 2001 26: S2–S12
Ditunno J et al. Recovery of upper-extremity strength in complete and incomplete tetraplegia: a multicenter study Arch Phys Med Rehab 2000 81: 389–393
Mange K, Ditunno J, Herbison G, Jaweed M . Recovery of strength at the zone of injury in motor complete and motor incomplete cervical spinal cord injured patients Arch Phys Med Rehab 1990 71: 562–565
Plotnik GD, Corretti MC, Vogel RA . Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal J Am Med Assoc 1997 278: 1682–1686
Hopman MTE, van Asten W, Oeseburg B . Changes in blood flow in the common femoral artery related to inactivity and muscle atrophy in individuals with long-standing paraplegia Adv Exp Med Biol 1996 388: 379–383
Shannon RP et al. Comparison of differences in the hemodynamic response to passive postural stress in healthy subjects <70 years and <30 years of age Am J Cardiol 1991 67: 1110–1116
Smith J et al. The effect of age on hemodynamic response to graded postural stress in normal men J Gerontol 1987 42: 406–411
Goldstein I, Shapiro D . The cardiovascular response to postural change as a function of race Biol Physiol 1995 39: 173–186
Nardo CJ et al. Descriptive epidemiology of blood pressure response to change in body position Hypertension 1999 33: 1123–1129
Creager M et al. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans J Clin Invest 1992 90: 1248–1253
Drexler H, Zeiher A, Meinzer K, Just H . Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolemic patients by L-arginine Lancet 1991 338: 1546–1550
Steinberg H et al. Type II diabetes abrogates sex differences in endothelial function in premenopausal women Circulation 2000 101: 2040–2046
Stornello M et al. Influence of glucose load on cardiovascular and humoral responses to a cold pressor test J Hum Hypertens 1995 9: 93–99
Fujimot Y et al. Decreased heart rate variability in patients with diabetes mellitus and ischemic heart disease Jpn Circ J 1996 60: 925–932
Steinberg H et al. Obesity/insulin resistance is associated with endothelial dysfunction J Clin Invest 1996 97: 2601–2610
Williams S et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus J Am Coll Cardiol 1996 27: 567–574
Shvartz E . Endurance fitness and orthostatic tolerance Aviat Space Environ Med 1996 67: 935–939
Talmadge R, Castro M, Apple Jr D, Dudley G . Phenotypic adaptations in human muscle fibres 6 and 24 wk after spinal cord injury J Appl Physiol 2002 92: 147–154
Kroese A . The effect of inactivity on reactive hyperemia in the human calf: a study with strain gauge plethysmography Scand J Clin Lab Invest 1977 37: 53–58
Kamiya A et al. Head-down bed rest alters sympathetic and cardiovascular responses to mental stress Am J Physiol Regul Integra Comp Physiol 2000 279: R440–R447
Miranda AR, Hassouna HI . Mechanisms of thrombosis in spinal cord injury Hematol/Oncol Clin North Am 2000 14: 401–416
Parker JL et al. Effects of exercise training on regulation of tone in coronary arteries and arterioles Med Sci Sports Exer 1994 26: 1252–1261
Houtman S, Colier W, Oeseburg B, Hopman M . Systemic circulation and cerebral oxygenation during head-up tilt in spinal cord injured individuals Spinal Cord 2000 38: 158–163
Houtman S, Oeseburg B, Hughson R, Hopman M . Sympathetic nervous system activity and cardiovascular homeostasis during head-up tilt in patients with spinal cord injuries Clin Auton Res 2000 10: 207–212
Levin B, Martin B, Natelson BH . Basal sympatho-adrenal function in quadriplegic man J Auton Nerv Sys 1980 2: 327–336
Shoemaker JK et al. Head-down-tilt bed rest alters forearm vasodilator and vasoconstrictor responses J Appl Physiol 1998 84: 1756–1762
Feinberg R et al. The ischemic window: a method for the objective quantitation of the training effect in exercise therapy for intermittent claudication J Vasc Sur 1992 16: 244–250
Cardillo C, CM K, Cannon R, Panza J . Attenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function Circulation 1999 99: 90–95
Dornyei G, Monos E, Kaley G, Koller G . Regular exercise enhances blood pressure lowering effect of acetylcholine by increased contribution of nitric oxide Acta Physiologica Hungarica 2000 87: 127–138
Hogan M, Richardson R, Kurdak S . Initial fall in skeletal muscle force development during ischemia is related to oxygen availability J Appl Physiol 1994 77: 2380–2384
Rochester L et al. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects, morphological and histochemical properties Paraplegia 1995 33: 514–522
Martin TP, Stein RB, Hoeppner PH, Reid DC . Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle J Appl Physiol 1992 72: 1401–1406
Acknowledgements
We would like to acknowledge Scott Bickel for his help in scheduling the testing of SCI subjects. Supported by Paralyzed Vetrans Association and NIH grants HL65179 and HD33738.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Olive, J., McCully, K. & Dudley, G. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord 40, 639–645 (2002). https://doi.org/10.1038/sj.sc.3101379
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101379
Keywords
This article is cited by
-
Predictors of lower extremity fracture-related amputation in persons with traumatic spinal cord injury: a case–control study
Spinal Cord (2023)
-
Increased risk of sensorineural hearing loss in patients with spinal cord injury: a nationwide longitudinal follow-up study
Spinal Cord (2021)
-
Intermittent negative pressure applied to the lower limb increases foot macrocirculatory and microcirculatory blood flow pulsatility in people with spinal cord injury
Spinal Cord (2018)
-
Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury
Spinal Cord (2007)
-
Cardiorespiratory responses during passive walking-like exercise in quadriplegics
Spinal Cord (2006)