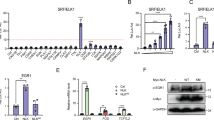
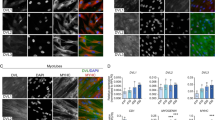
Abstract
In this study, we delineate the intracellular signalling pathways modulated by a conditional v-Src tyrosine kinase that lead to unrestrained proliferation and block of differentiation of primary avian myoblasts. By inhibiting Ras–MAPK kinase and phosphatidylinositol 3-kinase with different means, we find that both pathways play crucial roles in controlling v-Src-sustained growth factor and anchorage independence for proliferation. The Ras–MAPK kinase pathway also contributes to block of differentiation independently of cell proliferation since inhibition of this pathway both in proliferating and growth-arrested v-Src-transformed myoblasts induces expression of muscle-specific genes, fusion into multinucleated myotubes and assembly of specialized contractile structures. Importantly, we find that the p38 MAPK pathway is inhibited by v-Src in myoblasts and its forced activation results in growth inhibition and expression of differentiation, indicating p38 MAPK as a critical target of v-Src in growth transformation and myogenic differentiation. Furthermore, we show that downregulation of p38 MAPK activation may occur via Ras–MAPK kinase, thus highlighting a cross-regulation between the two pathways. Finally, we report that the simultaneous inhibition of MAPK kinase and calpain, combined to activation of p38 MAPK, are sufficient to reconstitute largely the differentiation potential of v-Src-transformed myoblasts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Aftab DT, Kwan J, Martin GS . (1997). Ras-independent transformation by v-Src. Proc Natl Acad Sci USA 94: 3028–3033.
Alemà S, Tató F . (1994). Oncogenes and muscle differentiation: multiple mechanisms of interference. Semin Cancer Biol 5: 147–156.
Bennett AM, Tonks NK . (1997). Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278: 1288–1291.
Boguski MS, McCormick F . (1993). Proteins regulating Ras and its relatives. Nature 366: 643–654.
Carragher NO, Frame MC . (2002). Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol 34: 1539–1543.
Castellani L, Reedy MC, Gauzzi MC, Provenzano C, Alemà S, Falcone G . (1995). Maintenance of the differentiated state in skeletal muscle: activation of v-Src disrupts sarcomeres in quail myotubes. J Cell Biol 130: 871–885.
Castellani L, Salvati E, Alemà S, Falcone G . (2006). Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J Biol Chem 281: 15249–15257.
Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN et al. (1997). Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276: 1848–1850.
Cowley S, Paterson H, Kemp P, Marshall CJ . (1994). Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77: 841–852.
Cuenda A, Cohen P . (1999). Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem 274: 4341–4346.
Dorman CM, Johnson SE . (1999). Activated Raf inhibits avian myogenesis through a MAPK-dependent mechanism. Oncogene 18: 5167–5176.
Falcone G, Ciuffini L, Gauzzi MC, Provenzano C, Strano S, Gallo R et al. (2003). v-Src inhibits myogenic differentiation by interfering with the regulatory network of muscle-specific transcriptional activators at multiple levels. Oncogene 22: 8302–8315.
Falcone G, Provenzano C, Alemà S, Tató F . (1992). Transformation of NIH3T3 cells by Rous sarcoma virus occurs with high efficiency in the absence of proviral rearrangements or amplification. Oncogene 7: 1913–1920.
Faust D, Dolado I, Cuadrado A, Oesch F, Weiss C, Nebreda AR et al. (2005). p38alpha MAPK is required for contact inhibition. Oncogene 24: 7941–7945.
Frame MC . (2002). Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602: 114–130.
Gallo R, Serafini M, Castellani L, Falcone G, Alemà S . (1999). Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol Biol Cell 10: 3137–3150.
Gredinger E, Gerber AN, Tamir Y, Tapscott SJ, Bengal E . (1998). Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J Biol Chem 273: 10436–10444.
Hattori S, Clanton DJ, Satoh T, Nakamura S, Kaziro Y, Kawakita M et al. (1987). Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol 7: 1999–2002.
Jiang BH, Zheng JZ, Vogt PK . (1998). An essential role of phosphatidylinositol 3-kinase in myogenic differentiation. Proc Natl Acad Sci USA 95: 14179–14183.
Keren A, Tamir Y, Bengal E . (2006). The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol 252: 224–230.
Lassar AB, Thayer MJ, Overell RW, Weintraub H . (1989). Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell 58: 659–667.
Li R, Zhou RP, Duesberg P . (1996). Host range restrictions of oncogenes: myc genes transform avian but not mammalian cells and mht/raf genes transform mammalian but not avian cells. Proc Natl Acad Sci USA 93: 7522–7527.
Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P . (2006). Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16: 36–44.
Marais R, Light Y, Paterson HF, Marshall CJ . (1995). Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J 14: 3136–3145.
Martin GS . (2001). The hunting of the Src. Nat Rev Mol Cell Biol 2: 467–475.
Nguyen KT, Wang WJ, Chan JL, Wang LH . (2000). Differential requirements of the MAP kinase and PI3 kinase signaling pathways in Src- versus insulin and IGF-1 receptors-induced growth and transformation of rat intestinal epithelial cells. Oncogene 19: 5385–5397.
Odajima J, Matsumura I, Sonoyama J, Daino H, Kawasaki A, Tanaka H et al. (2000). Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator for cell survival. J Biol Chem 275: 24096–24105.
Olson EN . (1992). Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol 154: 261–272.
Parsons SJ, Parsons JT . (2004). Src family kinases, key regulators of signal transduction. Oncogene 23: 7906–7909.
Penuel E, Martin GS . (1999). Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell 10: 1693–1703.
Perry RL, Parker MH, Rudnicki MA . (2001). Activated MEK1 binds the nuclear MyoD transcriptional complex to repress transactivation. Mol Cell 8: 291–301.
Puri PL, Sartorelli V . (2000). Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J Cell Physiol 185: 155–173.
Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ . (1996). MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255.
Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P et al. (1997). Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89: 457–467.
Russo S, Tató F, Grossi M . (1997). Transcriptional down-regulation of myogenin expression is associated with v-ras-induced block of differentiation in unestablished quail muscle cells. Oncogene 14: 63–73.
Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y et al. (2006). A common signalling cascade may underlie ‘addiction’ to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell 10: 425–435.
Smith MR, DeGudicibus SJ, Stacey D . (1986). Requirement for c-ras proteins during viral oncogene transformation. Nature 320: 540–543.
Sundaresan P, Farndale RW . (2002). p38 mitogen-activated protein kinase dephosphorylation is regulated by protein phsphatase 2A in human platelets activated by collagen. FEBS Lett 528: 139–144.
Thomas SM, Brugge JS . (1997). Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609.
Tureckova J, Wilson EM, Cappalonga JL, Rotwein P . (2001). Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J Biol Chem 276: 39264–39270.
Verderame MF, Kaplan JM, Varmus HE . (1989). A mutation in v-src that removes a single conserved residue in the SH-2 domain of pp60v-src restricts transformation in a host-dependent manner. J Virol 63: 338–348.
Yeatman TJ . (2004). A renaissance for SRC. Nat Rev Cancer 4: 470–480.
Weyman CM, Ramocki MB, Taparowsky EJ, Wolfman A . (1997). Distinct signaling pathways regulate transformation and inhibition of skeletal muscle differentiation by oncogenic Ras. Oncogene 14: 697–704.
Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR et al. (2000). p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol 20: 3951–3964.
Acknowledgements
We thank H Paterson for advice and reagents and all the colleagues who generously provided plasmids and antibodies. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (to SA); MIUR-FIRB (to GF and to SA) and FAR (ex 60%), Università di Cassino (to LC).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ciuffini, L., Castellani, L., Salvati, E. et al. Delineating v-Src downstream effector pathways in transformed myoblasts. Oncogene 27, 528–539 (2008). https://doi.org/10.1038/sj.onc.1210665
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210665
Keywords
This article is cited by
-
Phase I and pharmacokinetics/pharmacodynamics study of the MEK inhibitor RO4987655 in Japanese patients with advanced solid tumors
Investigational New Drugs (2015)