Abstract
Several studies have shown that schizophrenic patients and their biological relatives generate a greater number of leading saccades during smooth pursuit eye movement (SPEM) tasks. This abnormality may reflect a failure of cortical and/or cerebellar areas to coordinate saccadic and pursuit eye movements during visual tracking. The pharmacology of this phenomenon is not known. Here, we sought to replicate and extend the findings of Olincy et al (1998), who found that nicotine transiently reduced the number of leading saccades during SPEMs. A total of 27 subjects with schizophrenia (17 males; 14 smokers), and 25 healthy comparison subjects (nine males; 14 smokers) completed an eye-tracking task after receiving a 1.0 mg nasal spray of nicotine and during drug-free conditions. Results confirm that nicotine reduces the number of leading saccadic eye movements during visual tracking in schizophrenic patients. Baseline impairments and the beneficial effects of nicotine were not restricted to patient smokers, as nonsmoker patients exhibited the greatest number of leading saccades in the no drug condition and exhibited the most pronounced improvements after nicotine administration. Improvement in patient nonsmokers was not a function of previous smoking history. No effect of nicotine was observed in control nonsmokers. In contrast to the previous study, nicotine appeared to improve performance in control smokers. Overall, the study results support a functional role of nACh receptors in improving eye-tracking performance, and are consistent with the hypothesis, articulated by several investigators, that nACh receptor system abnormalities are responsible for a number of schizophrenia-related neurophysiological deficits.
Similar content being viewed by others
INTRODUCTION
Smooth pursuit eye movements (SPEMs) represent a highly developed and complex behavioral response to moving stimuli, which is served by a widely distributed neuronal network (Leigh and Zee, 1999). The SPEM response broadly involves: (a) the processing of retinal motion information (ie the movement of a target image on the retina); (b) the initiation of an oculomotor response based on retinal information, which can be modified by previous target motion information (Lisberger et al, 1987; Barnes et al, 2000); (c) the processing and integration of extraretinal motion signals generated by movement of the eyes (Turano and Heidenreich, 1999; Turano and Massof, 2001); and (d) the maintenance of pursuit based on a combination of predictive eye movements guided by extraretinal signals and corrective eye movements guided by retinal velocity and position error signals (Barnes and Asselman, 1991; van den Berg, 1988). Basic researchers have carefully described many of these processes and the neuronal substrates that underlie them in healthy human subjects (eg Braun et al, 1996; Petit et al, 1997; Berman et al, 1999; Schmid et al, 2001) and nonhuman primates (eg Lisberger and Movshon, 1999; Newsome et al, 1988; MacAvoy et al, 1991; Assad and Maunsell, 1995; Komatsu and Wurtz, 1989; Krauzlis, 2001; Suh et al, 2000).
Behavioral studies of SPEM suggest that several components of the response may be affected in schizophrenic patients. Abnormalities include (1) a failure of eye velocity to match target velocity during sustained visual tracking (ie low closed-loop gain) (eg Clementz and McDowell, 1994; Sweeney et al, 1999; Levy et al, 2000; see Levy et al, 1993 for a review), which is thought to reflect deficits in predictive components of the SPEM response (Thaker et al, 1996, 1999); (2) an increase in catch-up saccades, which is secondary to low gain (Abel and Ziegler, 1988; Sweeney et al, 1994); (3) decreased acceleration during pursuit initiation (Clementz et al, 1995; Ross et al, 1996), which may reflect retinal motion processing and/or anticipatory learning deficits (Chen et al, 1999; Avila et al, 2002a); and (4) an increased frequency of leading saccadic eye movements (Avila et al, 2002b; Levy et al, 2000; Ross et al, 2000), which may reflect a loss of cortical inhibitory control over saccades during pursuit (Litman et al, 1994; Ross et al, 1998; Lencer et al, 1999a).
Although hypotheses about the specific brain regions underlying these deficits can be generated based on knowledge of basic circuitry (eg predictive pursuit deficits implicate a circuit involving frontal eye fields (FEFs), mediosuperior temporal cortex (MST), posterior parietal cortex (PPC), and cerebellum), it is unclear what the functional abnormalities may be. Experimental administration of a particular pharmacological agent is one means of identifying receptor systems of interest, and in fact a number of drug-probe studies involving SPEM performance in schizophrenia have been conducted (eg Thaker et al, 1991; Friedman et al, 1992; Litman et al, 1994; Radant et al, 1998; Weiler et al, 2000; Avila et al, 2002c). Results of several drug-probe studies involving nicotine suggest that neuronal nicotinic receptors (nAChRs) may be involved (Olincy et al, 1998, 2003; Sherr et al, 2002; Depatie et al, 2002). Olincy et al (1998) found a trend towards improved closed-loop gain in patients after ad libitum smoking, and no effect of smoking in healthy smokers. We reported a similar effect of 1.0 mg of nicotine administered via nasal inhaler on closed-loop gain in patients who were both smokers and nonsmokers (Sherr et al, 2002). Depatie et al (2002) found that administration of nicotine via transdermal patch increased pursuit gain under normal tracking conditions, but not when target monitoring, an attention-enhancing pursuit task, was used.
Olincy et al (1998) also reported that ad libitum smoking reduced the frequency of leading saccades during pursuit in patients with schizophrenia, but not in healthy volunteers. Several studies have shown that patients with schizophrenia and at-risk relatives exhibit greater numbers of leading saccades during SPEM performance (Whicker et al, 1985; Rosenberg et al, 1997; Ross et al, 1998, 1999a, 2000; Levy et al, 2000; Avila et al, 2002b). The biological significance of this phenomenon and the role that nACh receptor systems play is not well understood, but it has been suggested that leading saccades reflect a failure of frontal–thalamic–cerebellar circuitry to coordinate saccadic eye movements during pursuit (Ross et al, 1998). One possibility, described by Olincy et al, is that cortical neurons in the prefrontal cortex fail to inhibit the generation of inappropriate saccades by the superior colliculus (SC), and that smoking among patients, in addition to having effects on α7-nAChR-mediated sensory gating (Adler et al, 1993, 1998), is an attempt to modify abnormalities in prefrontal activity (Olincy et al, 1998). Based on animal studies, George et al (2000) and others (eg Levin et al, 1996) have suggested that nicotinic agonists can enhance prefrontal functioning in schizophrenic patients by facilitating mesoprefrontal dopamine activity via activation of α7- and α4β2-containing nACh receptors.
Additional evidence for a role of nAChRs in modifying leading saccadic eye movements comes from Avila et al (2002c), who found that a 0.1 mg/kg bolus injection of ketamine specifically increased the number of leading saccades during SPEM performance in healthy volunteers to a level similar to that observed in first-degree relatives of patients with schizophrenia exhibiting schizophrenia spectrum personality symptoms. Ketamine, in addition to blocking N-methyl-D-aspartate (NMDA) receptors, has been shown to block α7 and α4β2nAChR receptors (Coates and Flood, 2001; Hilmas and Albuquerque, 2002).
Interest in the functional significance of schizophrenia-related increases in leading saccades during pursuit is fairly recent, and there are therefore a limited number of studies examining the phenomenon. Olincy et al (1998) are the only investigators to examine the possible effects of nicotine on leading saccades in patients with schizophrenia. We therefore sought to replicate and extend their findings, using data from Sherr et al (2002) to examine the effects of nicotine on leading saccades in smokers and nonsmokers with and without schizophrenia. The inclusion of smokers and nonsmokers gives the opportunity to assess the influence of smoking status on baseline and nicotine condition leading saccade measures. By including nonsmokers, nicotine effects can also be examined independent of potential nicotine withdrawal effects and group differences in receptor changes related to chronic tobacco use (Breese et al, 2000). The use of a controlled dose of nicotine (1.0 mg administered via nasal inhaler) also avoids potential problems with differences in nicotine dose and the introduction of other centrally active compounds, which may be associated with ad libitum smoking procedures.
METHODS
Research Participants
Patients with schizophrenia were recruited from in-patient and outpatient programs at the Maryland Psychiatric Research Center (MPRC). Control subjects were drawn from the MPRC-Intervention Research Center (IRC) healthy control pool, which recruits subjects from the greater Baltimore/Washington metropolitan area using newspaper advertisements.
Clinical assessments
Patient diagnoses were confirmed using the Structured Clinical Interview for DSM-IV diagnosis (SCID-IV) (First et al, 1997). The SCID-IV, Structured Interview for DSM-IV Personality Disorders (SID-P) (Pfohl et al, 1989), and Family History Research Diagnostic Criteria (FH-RDC) (Andreasen et al, 1986) were used to screen and exclude control subjects with a family history of psychotic illness, current or lifetime Axis-I, or -II disorders (including substance dependence), or a history of substance abuse in the 6 months prior to study participation. Patient and community volunteers who had suffered a heart attack within the past year, or had chronic obstructive lung disease and/or pulmonary emphysema, pre-existing clinically significant cardiovascular disease, or a neurological condition were not eligible to participate. All patient volunteers were on stable doses of antipsychotic medications at the time of testing; three (10%) were taking typical neuroleptics, nine (31%) clozapine, one (3%) risperidone, 14 (48%) olanzapine, and two (6%) were taking olanzapine and haloperidol. Sociodemographic and smoking-related characteristics of the sample are shown in Table 1.
Laboratory Procedures
Subjects abstained from cigarettes for 2 h prior to testing and participated in two randomly ordered testing sessions: a baseline in which no drug was given and after nicotine administration. A 1.0 mg dose of nicotine was administered via Nicotrol-Nasal Spray (McNeil) (two 0.5 mg puffs in each nostril) 5 min before eye movement testing. Participants also completed a cognitive battery (divided into two sessions) under baseline and nicotine conditions. These results are described in a separate report (Myers et al, 2002). Thus, subjects received three separate 1.0 mg doses (one during eye movement testing, and two during cognitive testing). The order of the testing sessions was counterbalanced within nicotine and no drug conditions to control for order effects and to insure that regression to the mean did not systematically affect any one condition.
Oculomotor Data Acquisition
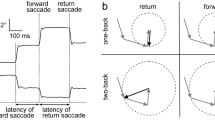
The ramp–mask–ramp task used in the study has been described previously (Thaker et al, 1998; Sherr et al, 2002). Briefly, a foveal–petal step-ramp was presented followed by target motion in a horizontal plane, back and forth, at a constant velocity. After approximately two to three sweeps across the monitor, the target was unpredictably masked for 500 ms. Analysis and results of eye movements during initiation, sustained visual tracking, and target-masking are described in Sherr et al (2002). Here, we describe leading saccadic eye movements occurring during visible target motion. Target presentations were carried out in three blocks of 12 trials each for no drug and nicotine conditions. Two target speed trials (9.4 and 18.7°/s) were included in each block. Each trial consisted of four to five sweeps and one mask. The order of the trials within a block was randomized. Each block lasted approximately 3.5 min. Here, we focus on data for 18.7°/s trials in order to allow comparisons with the previously published report on leading saccades and smoking, which used a target speed of 16°/s (Olincy et al, 1998). In addition, a number of studies suggest that SPEM deficits are more reliably observed when higher target speeds are used (eg Abel et al, 1991; Clementz and McDowell, 1994; Clementz et al, 1995; Thaker et al, 1998)—this includes a report by Lencer et al (1999b) who found group differences in leading saccades only at higher target speeds (15 vs 30°/s).
Eye movement data were obtained using infrared oculography (500 Hz sampling rate limited by a 4 ms time constant). Data were digitized using a 16-bit analog-to-digital converter. Digital data were filtered off-line using a low-pass filter (cutoff 75 Hz). Eye movements were analyzed blind to drug condition using interactive software. Inter-rater reliability estimates (ICCs) for scoring routines are maintained above 0.95.
Leading saccade measures
Blinks were identified based on characteristic morphology and removed. Saccades were identified by computer algorithm based on velocity (>35°/s) and acceleration (>600°/s2) criteria and verified by visual inspection. Saccades occurring within 130 ms of changes in the target direction were not included. Saccades were classified as leading if they: (1) occurred in the direction of target motion; (2) either began and ended ahead of the target or, if beginning behind the target, moved the eyes to a position ahead of the target resulting in a position error equal to or greater than the original position error; and (3) were followed by a period, at least 50 ms in duration, of postsaccadic slowing (defined as the eye velocity 50% of target velocity for 18.7°/s targets and eye velocity 75% of target velocity for 9.4°/s targets). In addition, an amplitude criterion of >0.75°<5.0° visual angle was employed. Criteria based on amplitude, position error, and postsaccadic slowing come from Ross and others (Radant and Homer, 1992; Ross et al, 1999b, 2001). Across groups and drug conditions, the average amplitude of saccades identified as leading was 2.12±1.07. Leading saccades were assessed using a ratio of time spent in leading saccades to time spent in pursuit. This is similar to the percentage of total distance due to leading saccades used by Ross et al (1999a) and is designed to assess what percentage of visual tracking is accomplished through leading saccadic eye movements.
Statistical Analyses
Baseline differences were examined using a 2 × 2 ANOVA with smoking status and diagnosis as between-subject factors. Effects of nicotine on the leading saccade/pursuit ratio (LSPR) were assessed using a mixed design repeated measures ANOVA. The model included terms for diagnosis (patient vs control), smoking status (smoker vs nonsmoker), and the diagnosis by smoking status interaction. Three-way interactions were followed by separate 2 × 2 ANOVAs and probed using simple effects analysis (Levine, 1991).
RESULTS
Means, standard deviations, and effect size estimates for no drug and nicotine conditions in patient and control smokers and nonsmokers are given in Table 2. The mean LSPR values in patients and controls (smokers and nonsmokers combined) were 1.6±1.3 and 1.2±0.9, respectively—similar to previously reported values (Avila et al, 2002b). Analysis of baseline differences yielded a smoking status by diagnosis interaction (F(1,48)=4.75, p<0.05). Means are shown in Table 2 and the left panel of Figure 1. Patient nonsmokers exhibited significantly higher LSPR values compared with control nonsmokers (p<0.05). No other significant baseline differences were observed. The mixed design ANOVA yielded a significant drug by diagnosis by smoking status interaction (F(1,48)=4.55, p<0.05). This three-way interaction is shown in Figure 1. Two-way mixed design ANOVAs (drug by diagnosis) were then performed separately for smokers and nonsmokers.
Nonsmokers
Analysis of LSPR among nonsmokers revealed a significant drug by diagnosis interaction (F(1,22)=4.42, p<0.05). Analysis of simple effects (examining the effect of drug within each group) showed that nicotine significantly reduced the LSPR among patient nonsmokers (p<0.025), whereas control nonsmokers did not change (p>0.70). Analysis of simple effects (examining group differences in no drug and nicotine conditions) showed that nicotine normalized performance in patient nonsmokers (ie eliminated group differences at baseline; p-valuebaseline<0.05, p-valuenicotine>0.20).
Smokers
For patient and control smokers, there was a significant main effect of drug (F(1,26)=7.95, p<0.005). Mean LSPRs for baseline vs nicotine conditions (collapsed across diagnosis) were 1.43±1.12 and 0.94±0.65, respectively. Both patients and control smokers exhibited decreased LSPRs after nicotine compared with the no drug condition. Analysis of simple effects showed that this change was statistically significant in control subjects (p<0.05), but statistically not significant in the patient group (p<0.20). Effect size estimates (Cohen's d) for no drug/nicotine changes were 0.63 and 0.46 in the control and patient groups, respectively. Analysis of simple effects (examining group differences within each drug condition) showed that patients and controls were not different in no drug and nicotine conditions (p-values>0.58)—the latter, reflecting a similar magnitude of change among patients.
Figure 2 shows individual subjects' values for LSPR in no drug and drug conditions. Changes in LSPR among patient nonsmokers did not appear related to a previous history of tobacco use. Only three of 13 patient nonsmokers reported having smoked in the past. Of these, two patients exhibited modest changes and one patient markedly improved (see subjects labeled PS4–6 in Figure 2). Changes in LSPR among control and patient smokers were not significantly correlated with FTND scores, average number of cigarettes smoked per day, or the number of years a subject smoked. Study results remained the same when the number of leading saccades was used as the dependent measure (data not shown). Note that baseline differences and the direction of change after nicotine administration were similar for 9.4°/s targets to those reported here but not statistically significant. This is consistent with a previous report showing reliable differences only at higher target speeds (Lencer et al, 1999b).
Changes in LSPR for individual subjects. ‘PS’ designates nonsmokers who previously smoked. *Drug by diagnosis by smoking status, drug by diagnosis (within smokers), and associated simple effects analyses were conducted removing the identified case. Although contributing to the magnitude of the effects, removal of this case did not change the pattern of results. Note that drug order was counterbalanced in order to control for order effects and to insure that regression to the mean did not systematically affect any one condition.
DISCUSSION
Consistent with what has been previously reported by Olincy et al (1998), we found evidence that nicotine reduces the number of leading saccades that occur during pursuit of a moving target among patients with schizophrenia. Patients as a group went from a mean LSPR of 1.61±1.38 to 1.06±0.61 (p=0.03) after a 1.0 mg dose of nicotine (Cohen's d=0.56). Evidence of drug effects in the previous study was based on ad libitum smoking, which potentially involves a number of centrally active compounds. In contrast, our results are based on nicotine nasal-spray, and therefore can be used to confirm the putative role of nicotine in reducing leading saccades during SPEMs.
Analysis of drug effects by smoking status revealed that changes were more robust among patient nonsmokers. The modest effect of nicotine among patient smokers is somewhat inconsistent with the previous study, which reported an effect size greater than 1.00 (based on the reported paired t-test value). This is compared with an effect size of 0.46 for patient smokers in the present study. Task parameters, including target velocity, were similar in the two studies and not likely to explain the difference. However, differences in the duration of withdrawal and nicotine dose may account for the greater magnitude of change observed by Olincy et al. The average length of withdrawal in the previous study was 10 h, compared with 2 h in the present study. Our results indicate that nicotine does lower the LSPR—leaving open the possibility that additional time was needed for patient smokers to return to ‘true’ baseline values (which would be expected to be more similar to the baseline values observed for patient nonsmokers). The previous study also employed a higher dose of nicotine based on the number of cigarettes smoked during the ad libitum session (nine of the 15 patients in the previous study smoked two or more cigarettes). Thus, a higher dose may have been needed to achieve a similar magnitude of change in smokers.
This is the first study to examine the effects of nicotine on leading saccades in schizophrenic patients who do not smoke. We observed a significant effect of nicotine in this group, which was sufficient to eliminate patient-control baseline differences. This effect cannot be attributed to a past history of tobacco use, as most of the patient nonsmokers had never smoked. Numerous authors have suggested that the high prevalence of smoking among patients is due in large part to the presence of neurophysiological abnormalities involving neuronal nicotinic receptor systems (Leonard et al, 2001; Stassen et al, 2000). The fact that nicotine improved the eye-tracking performance of patient nonsmokers suggests that the presence of these deficits is not necessarily sufficient to lead a patient to smoke. This underscores the complex set of factors that lead to tobacco use in general, and among patients with schizophrenia in particular.
Results from control subjects conflict somewhat with what has been previously observed. We found that control nonsmokers exhibited the lowest LSPR values at baseline and were unaffected by nicotine. This is what Olincy et al observed for control smokers. However, control smokers in the current study exhibited baseline LSPR values similar to those observed among patient smokers and showed significant improvement after nicotine administration. This did not appear to be the result of outlying data (see Figure 2). Elevated baseline values, relative to control nonsmokers, are consistent with studies suggesting that smoking among healthy individuals can be associated with poor performance on some neurocognitive assessments. However, one would not expect nicotine-induced improvements under these conditions. It is not clear to what degree possible withdrawal effects and/or patient-control differences in receptor changes associated with chronic tobacco use may explain these results. Note that post-mortem data have shown that nonschizophrenic smokers show increased nicotinic receptor binding in the hippocampus, cortex, and caudate consistent with expected receptor upregulation after chronic tobacco use, while schizophrenic patient smokers do not show the expected increases (Breese et al, 2000). In this regard, data from nonsmokers are particularly important as they are unaffected by these factors. Inclusion of both control smokers and nonsmokers in future studies may help to resolve the discrepancy.
Neurophysiological Basis of Leading Saccades
The biological significance of leading saccadic eye movements during visual tracking is not well understood. One of the primary features of these saccadic eye movements, hypermetria, or target overshoot, is most often associated with cerebellar lesions (Robinson, 1995; Takagi et al, 1998; Quaia et al, 1999; Leigh and Zee, 1999). Thus, one possibility is that leading saccades represent hypermetric catch-up saccades caused by subtle abnormalities in cerebellar functioning. This interpretation is consistent with a previously reported negative correlation between leading saccades and pursuit gain (Radant and Hommer, 1992). We have also observed a modest negative correlation between pursuit gain and LSPR in a sample of schizophrenic patients (n=66; r=0.39, p<0.05, unpublished data). Arguing against this interpretation is the fact that saccadic hypermetria is not observed for visually guided saccades (eg Karoumi et al, 1998; Maruff et al, 1998), and leading saccades can occur when the eyes are close to or ahead of the target, a situation where position error signals are inconsistent with the generation of catch-up saccades. Thus, leading saccades appear to have both spatial and temporal dysmetric components, and are therefore more likely to reflect abnormalities in the integration of saccadic and pursuit eye movements during visual tracking rather than simple saccadic hypermetria. This is consistent with previous descriptions of leading saccades as ‘context-inappropriate’ or ‘intrusive’ saccades, which may be related to loss of inhibitory control over saccadic eye movements during pursuit (Litman et al, 1994; Ross et al, 1996; Lencer et al, 1999a).
A model proposed by Ross et al (1996), (1998) suggests a failure of cerebellar neurons to coordinate saccadic and pursuit signals received by cortical structures such as the FEFs via a fronto-thalamic-cerebellar circuit.
This shares a number of features with a model of cerebellar gain learning described by Gancarz and Grossberg (1999). According to Gancarz and Grossberg, saccadic control can be achieved by cerebellar adjustment of sampling signals received from SC, visual/posterior parietal cortex (VC/PPC), and FEF via the nucleus reticularis tegmenti pontis (NRTP) and pontine nuclei. Weights are adjusted by error signals carried by cerebellar climbing fibers originating in inferior olive (IO). They suggest that sampling streams originating from SC, VC/PPC, and FEF and their respective weights are associated with reactive, attentive, and planned aspects of saccadic eye movements, respectively, and that these signals compete through mutual inhibition that tends to favor attentive and planned streams. This model suggests that diminished cortical input associated with attentive and/or planned components could lead to inappropriate weighting of reactive signals favoring the appearance of intrusive saccades. Alternatively, abnormalities could arise from a failure of cerebellar neurons to integrate these signals properly. Interestingly, Van Gelder et al (1995) observed a decrease in leading saccades when healthy individuals were asked to analyze some changing feature of the pursuit target, a procedure that is thought to enhance attentional processes. This parallels Depatie et al's (2002) pursuit gain findings showing that nicotine had an affect on eye-tracking performance under normal, but not under attention enhancing, conditions. These data suggest that pursuit related attentional processes might play a role in some nicotine-induced changes in SPEM performance. The effect of target analysis on leading saccades in schizophrenic patients needs to be examined.
In humans, both the frontal and parietal cortex show moderate levels of [3H]nicotine binding. A moderate level of [3H]epibatidine binding is also observed in the parietal cortex and cerebellum. Both ligands are associated with high-affinity nicotinic receptors such as α4β2. Lower levels of [125I]α-bungarotoxin binding sites, which are associated with α7nACh receptors, are seen in both the cortex and cerebellum (Patterson and Nordberg, 2000). The exact role of nACh receptors in these regions, and how they may participate in the circuit described above is not clear. In general, a number of these receptor subtypes are located presynaptically where they facilitate the release of a number of neurotransmitters including glutamate, GABA, and dopamine (Brioni et al, 1997; Patterson and Nordberg, 2000). Additional experiments examining the behavioral effects of ligands specific to particular receptor subtypes are needed.
SUMMARY
Study results confirm that nicotine reduces the number of leading saccadic eye movements and improves eye-tracking performance in schizophrenic patients. Baseline impairments and the beneficial effects of nicotine are not restricted to patient smokers, as nonsmokers exhibited the greatest number of leading saccades in the no drug condition and exhibited the most pronounced improvements. This improvement was not a function of previous smoking history. Study results from patient smokers suggest that longer (>2 h) withdrawal periods and/or higher nicotine doses may be necessary to obtain accurate baseline performance levels and to induce significant changes in performance in patient smokers. Overall, the study results support a functional role of nACh receptors in improving eye-tracking performance, and are consistent with the hypothesis, articulated by several investigators, that nACh receptor system abnormalities are responsible for a number of schizophrenia-related neurophysiological deficits (Adler et al, 1998; Leonard et al, 2001; Thaker et al, 2002).
References
Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY (1991). Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol Psychiatry 29: 1063–1072.
Abel LA, Ziegler AS (1988). Smooth pursuit eye movements in schizophrenic—what constitutes quantitative assessment. Biol Psychiatry 24: 747–761.
Adler LE, Hoffer LD, Wiser A, Freedman R (1993). Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150: 1856–1861.
Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K et al (1998). Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24: 189–202.
Andreasen NC, Rice J, Endicott J, Reich T, Coryell W (1986). The family history approach to diagnosis. How useful is it? Arch Gen Psychiatry 43: 421–429.
Assad JA, Maunsell JH (1995). Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature 373: 518–521.
Avila MT, McMahon RP, Elliot AR, Thaker GK (2002b). Neurophysiological markers of vulnerability to schizophrenia: sensitivity and specificity of specific quantitative eye movement measures. J Abnorm Psychol 111: 259–267.
Avila MT, Sherr JD, Hong E, Myers C, Blaxton TA, Thaker GK (2002a). Can enhanced learning explain nicotine-induced improvements in pursuit initiation in patients with schizophrenia. Program No. 705.8. 2002 Abstract Viewer/Iternary Planner, Society for Neuroscience: Washington, DC, 2002, CD-ROM.
Avila MT, Weiler MA, Lahti AC, Tamminga CA, Thaker GK (2002c). Effects of ketamine on leading saccades during smooth pursuit eye movements may implicate cerebellar dysfunction in schizophrenia. Am J Psychiatry 159: 1490–1496.
Barnes GR, Asselman PT (1991). The mechanism of prediction in human smooth pursuit eye movements. J Physiol 439: 439–461.
Barnes GR, Barnes DM, Chakraborti SR (2000). Ocular pursuit responses to repeated, single-cycle sinusoids reveal behavior compatible with predictive pursuit. J Neurophysiol 84: 2340–2355.
Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulburn KR et al (1999). Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp 8: 209–225.
Braun DI, Boman DK, Hotson JR (1996). Anticipatory smooth eye movements and predictive pursuit after unilateral lesions in human brain. Exp Brain Res 110: 111–116.
Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM et al (2000). Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 23: 351–364.
Brioni JD, Decker MW, Sulliva JP, Arneric SP (1997). The pharmacology of (−)-nicotine and novel cholinergic channel modulators. Adv Pharmacol 37: 153–212.
Chen Y, Levy DL, Nakayama K, Mattysse S, Palafox G, Holzman PS (1999). Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Arch Gen Psychiatry 56: 155–161.
Clementz BA, McDowell JE (1994). Smooth pursuit in schizophrenia: abnormalities of open- and closed-loop responses. Psychophysiology 31: 79–86.
Clementz BA, Reid SA, McDowell JE, Cadenhead KS (1995). Abnormality of smooth pursuit eye movement initiation: specificity to the schizophrenia spectrum? Psychophysiology 32: 130–134.
Coates KM, Flood P (2001). Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha7 and alpha4beta2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol 134: 871–879.
Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN et al (2002). Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology 27: 1056–1070.
First MB, Spitzer RL, Gibbon M, Williams JW (1997). Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department: New York, NY.
Friedman L, Jesberger JA, Melzer HY (1992). Effect of typical antipsychotic medication and clozapine on smooth pursuit performance in patients with schizophrenia. Psychiatry Res 41: 25–36.
Gancarz G, Grossberg S (1999). A neural model of saccadic eye movement controls explains task-specific adaptation. Vision Res 39: 3123–3143.
George TP, Verrico CD, Picciotto MR, Roth RH (2000). Nicotinic modulation of mesoprefrontal dopamine neurons: pharmacologic and neuroanatomic characterization. J Pharmacol Exp Ther 295: 58–66.
Hilmas C, Albuquerque EX (2002). The psychotomimetic drugs phencyclidine and ketamine inhibit α7 and α4β2 nicotinic receptors: clinical implications. Program No. 242.16. 2002 Abstract Viewer/Iternary Planner, Society for Neuroscience: Washington, DC, 2002, CD-ROM.
Karoumi B, Ventre-Dominey J, Vighetto A, Darlery J, d'Amato T (1998). Saccadic eye movements in schizophrenia. Psychiatry Res 77: 9–19.
Komatsu H, Wurtz RH (1989). Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol 62: 31–47.
Krauzlis RJ (2001). Extraretinal inputs to neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 86: 2629–2633.
Leigh JR, Zee DS (1999). The Neurology of Eye Movements. F.A. Davis: Philadelphia, PA.
Lencer R, Malchow CP, Krecker K, Nolte A, Pinnow M, Zimmerman S et al (1999a). Smooth pursuit performance in families with multiple occurrences of schizophrenia and nonpsychotic families. Biol Psychiatry 45: 694–703.
Lencer R, Malchow CP, Trillenberg-Krecker K, Schwinger E, Arolt V (1999b). Eye-tracking dysfunction in families with sporadic and familial schizophrenia. Biol Psychiatry 47: 391–401.
Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C et al (2001). Smoking and mental illness. Pharmacol Biochem Behav 70: 561–570.
Levine G (1991). A Guide to SPSS for Analysis of Variance. Lawrence Erlbaum Associates: Hillsdale, NJ.
Levin ED, Wilson W, Rose JE, McEvoy J (1996). Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 15: 429–436.
Levy DL, Holzman PS, Mendell NR (1993). Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull 19: 462–501.
Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A et al (2000). Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr Res 42: 171–185.
Lisberger SG, Morris EJ, Tychsen L (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10: 97–129.
Lisberger SG, Movshon JA (1999). Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. J Neurosci 19: 2224–2246.
Litman RE, Hommer DW, Radant A, Clem T, Pickar D (1994). Quantitative effects of typical and atypical neuroleptics on smooth pursuit eye tracking in schizophrenia. Schizophr Res 12: 107–120.
MacAvoy MG, Gottlieb JP, Bruce CJ (1991). Smooth-pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex 1: 95–102.
Maruff P, Danckert J, Pantelis C, Currie J (1998). Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med 28: 1091–1100.
Myers CS, Robles O, Kakoyannis NA, Sherr JD, Blaxton TA, Thaker GK (2002). The beneficial effects of nicotine on long-term memory in schizophrenic patients. Biol Psychiatry submitted.
Newsome WT, Wurtz RH, Komatsu H (1988). Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol 60: 604–620.
Olincy A, Johnson LL, Ross RG (2003). Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Res 117: 223–236.
Olincy A, Ross RG, Young DA, Roath M, Freedman R (1998). Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology 18: 175–185.
Patterson D, Nordberg A (2000). Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61: 75–111.
Petit L, Clark VP, Ingeholm J, Haxby JV (1997). Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol 77: 3386–3390.
Pfohl B, Blum N, Zimmerman M, Stangl D (1989). Structured Interview for DSM-III-R Personality (SIDP-R). University of Iowa: Iowa.
Quaia C, Lefe‘vre P, Optican LM (1999). Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 82: 999–1018.
Radant AD, Bowdle AT, Cowley DS, Kharasch ED, Roy-Byrne PP (1998). Dose ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology 19: 434–444.
Radant AD, Hommer DW (1992). A quantitative analysis of saccades and smooth pursuit during visual pursuit tracking: a comparison of schizophrenics with normals and substance abusing controls. Schizophr Res 6: 225–235.
Robinson FR (1995). Role of the cerebellum in movement control and adaptation. Curr Opin Neurobiol 5: 755–762.
Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L (1997). Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Res 66: 121–130.
Ross RG, Olincy A, Harris JG, Sullivan B, Radant A (2000). Smooth pursuit eye movements in schizophrenia and attentional dysfunction: adults with schizophrenia, ADHD, and a normal comparison group. Biol Psychiatry 48: 197–203.
Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Freedman R (1998). Anticipatory saccades during smooth pursuit eye movements and familial transmission of schizophrenia. Biol Psychiatry 44: 690–697.
Ross RG, Olincy A, Harris JG, Radant A, Hawkins M, Adler LE et al (1999a). Evidence for bilineal inheritance of physiological indicators of risk in childhood-onset schizophrenia. Am J Med Genet 88: 188–199.
Ross RG, Olincy A, Radant A (1999b). Amplitude criteria and anticipatory saccades during smooth pursuit eye movements in schizophrenia. Psychophysiology 36: 464–468.
Ross RG, Olincy A, Zerbe G, Radant A (2001). Which duration of postsaccadic slowing identifies anticipatory saccades during smooth pursuit eye movements. Psychophysiology 38: 325–333.
Ross DE, Thaker GK, Buchanan RW, Lahti AC, Medoff D, Bartko JJ et al (1996). Association of abnormal smooth pursuit eye movements with the deficit syndrome in schizophrenic patients. Am J Psychiatry 153: 1158–1165.
Schmid A, Rees G, Frith C, Barnes G (2001). An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. NeuroReport 12: 1409–1414.
Sherr JD, Meyers C, Avila MT, Elliot A, Blaxton TA, Thaker GK (2002). The effects of nicotine on specific eye tracking measures in schizophrenia. Biol Psychiatry 52: 721–728.
Stassen HH, Bridler R, Hagele S, Hergersberg M, Mehmann B, Schinzel A et al (2000). Schizophrenia and smoking: evidence for a common neurobiological basis? Am J Med Genet 96: 173–177.
Suh M, Leung HC, Kettner RE (2000). Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. J Neurophysiol 84: 1835–1850.
Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ (1994). Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol 103: 222–230.
Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME (1999). Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry 46: 671–680.
Takagi M, Zee DS, Tamargo RJ (1998). Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931.
Thaker GK (2002). Current progress in schizophrenia research: sensory gating deficit in schizophrenia: is the nicotinic alpha-7 receptor implicated? J Nerv Ment Dis 190: 550–551.
Thaker GK, Ellsberry MM, Lahti A, Tamminga C (1991). Tobacco smoking increases square-wave jerks during pursuit eye movements. Biol Psychiatry 29: 82–88.
Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR (1999). Smooth pursuit eye movements to extraretinal motion signals: deficits in patients with schizophrenia. Psychiatry Res 88: 209–219.
Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim CE et al (1996). Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Res 59: 221–237.
Thaker GK, Ross DE, Cassady S, Adami HM, Laporte D, Medoff DR et al (1998). Smooth pursuit eye movements to extraretinal motion signals. Arch Gen Psychiatry 55: 830–836.
Turano KA, Heidenreich SM (1999). Eye movements affect the perceived speed of visual motion. Vision Res 39: 1177–1187.
Turano KA, Massof RW (2001). Nonlinear contribution of eye velocity to motion perception. Vision Res 41: 385–395.
van den Berg AV (1988). Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Exp Brain Res 72: 95–108.
Van Gelder P, Lebedev S, Liu PM, Tsui WH (1995). Anticipatory saccades in smooth pursuit: task effects and pursuit vector after saccades. Vision Res 35: 667–678.
Weiler MA, Thaker GK, Lahti AC, Tamminga CA (2000). Ketamine effects on eye movements. Neuropsychopharmacology 23: 645–653.
Whicker L, Abel LA, Dell'Osso LF (1985). Smooth pursuit eye movements in the parents of schizophrenics. Neuro-ophthalmology 5: 1–8.
Acknowledgements
The work presented was supported by NIH Grants MH49826 and MH40279, General Clinical Research Centers Grant # M01 RR 165001, and a Research Cooperation Agreement with Novartis. We acknowledge the contributions of Healthy Langley and John Schreiber, who assisted in collecting and processing the eye movement data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avila, M., Sherr, J., Hong, E. et al. Effects of Nicotine on Leading Saccades during Smooth Pursuit Eye Movements in Smokers and Nonsmokers with Schizophrenia. Neuropsychopharmacol 28, 2184–2191 (2003). https://doi.org/10.1038/sj.npp.1300265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300265
Keywords
This article is cited by
-
Effects of nicotine on smooth pursuit eye movements in healthy non-smokers
Psychopharmacology (2019)
-
Translational utility of rodent hippocampal auditory gating in schizophrenia research: a review and evaluation
Translational Psychiatry (2015)
-
Small effects of smoking on visual spatiotemporal processing
Scientific Reports (2014)
-
Smoking improves divided attention in schizophrenia
Psychopharmacology (2014)
-
Targeting the Nicotinic Cholinergic System to Treat Attention-Deficit/Hyperactivity Disorder: Rationale and Progress to Date
CNS Drugs (2014)