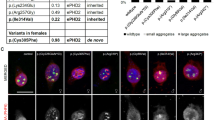
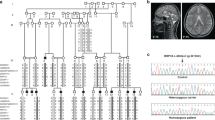
Abstract
Autism has a strong genetic background with a higher frequency of affected males suggesting involvement of X-linked genes and possibly also other factors causing the unbalanced sex ratio in the etiology of the disorder. We have identified two missense mutations in the ribosomal protein gene RPL10 located in Xq28 in two independent families with autism. We have obtained evidence that the amino-acid substitutions L206M and H213Q at the C-terminal end of RPL10 confer hypomorphism with respect to the regulation of the translation process while keeping the basic translation functions intact. This suggests the contribution of a novel, possibly modulating aberrant cellular function operative in autism. Previously, we detected high expression of RPL10 by RNA in situ hybridization in mouse hippocampus, a constituent of the brain limbic system known to be afflicted in autism. Based on these findings, we present a model for autistic disorder where a change in translational function is suggested to impact on those cognitive functions that are mediated through the limbic system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Volkmar FR, Pauls D . Autism. Lancet 2003; 362: 1133–1141.
Klauck SM . Genetics of autism spectrum disorder. Eur J Hum Genet 2006; 14: 714–720.
Belmonte MK, Cook Jr EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry 2004; 9: 646–663.
Raymond GV, Bauman ML, Kemper TL . Hippocampus in autism: a Golgi analysis. Acta Neuropathol (Berlin) 2003; 91: 117–119.
Fombonne E . Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 2003; 33: 365–382.
Smalley SL . Genetic influences in childhood-onset psychiatric disorders: autism and attention-deficit/hyperactivity disorder. Am J Hum Genet 1997; 60: 1276–1282.
Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Råstam M, Sponheim E et al. Genome-wide scan for autism susceptibility genes. Hum Mol Genet 1999; 8: 805–812.
Auranen M, Vanhala R, Varilo T, Ayers K, Kempas E, Ylisaukko-oja T et al. A genomewide screen for autism-spectrum disorders: evidence for a major susceptibility locus on chromosome 3q25–27. Am J Hum Genet 2002; 71: 777–790.
Shao Y, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA et al. Genomic screen and follow-up analysis for autistic disorder. Am J Med Genet (Neuropsychiatr Genet) 2002; 114: 99–105.
Yonan AL, Alarcon M, Cheng R, Magnusson PKE, Spence SJ, Palmer AA et al. A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet 2003; 73: 886–897.
Vincent JB, Melmer G, Bolton PF, Hodgkinson S, Holmes D, Curtis D et al. Genetic linkage analysis of the X chromosome in autism, with emphasis on the fragile X region. Psychiatr Genet 2005; 15: 83–90.
Kolb-Kokocinski A, Mehrle A, Bechtel S, Simpson JC, Kioschis P, Wiemann S et al. The systematic functional characterisation of Xq28 prioritises candidate disease genes. BMC Genomics 2006; 7: 29.
Fombonne E . Modern views of autism. Can J Psychiatry 2003; 48: 503–505.
Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G et al. Structure of the 80S ribosome from Saccaromyces cerevisiae-tRNA-ribosome and subunit-subunit interactions. Cell 2001; 107: 373–386.
Nissan TA, Baßler J, Petfalski E, Tollervey D, Hurt E . 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J 2002; 21: 5539–5547.
Gadal O, Straub D, Kessl J, Trumpower B, Tollervey D, Hurt E . Nuclear export of 60S ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol Cell Biol 2001; 21: 3405–3415.
West M, Hedges JB, Chen A, Johnson AW . Defining the order in which Nmd3p and Rpl10p load onto nascent 60S ribosomal subunits. Mol Cell Biol 2005; 25: 3802–3813.
Eisinger DP, Dick FA, Trumpower BL . Qsr1p, a 60S ribosomal subunit protein, is required for joining of 40S and 60S subunits. Mol Cell Biol 1997; 17: 5136–5145.
Klauck SM, Poustka F, Benner A, Lesch K-P, Poustka A . Serotonin transporter (5-HTT) gene variants associated with autism? Hum Mol Genet 1997; 6: 2233–2238.
Lord C, Rutter M, Le Couteur A . Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24: 659–685.
Lord C, Risi S, Lambrecht L, Cook Jr EH, Leventhal BL, DiLavore PC et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30: 205–223.
Sparrow SS, Balla DA, Cicchetti DV (eds). Vineland Adaptive Behavior Scales. AGS Publishing: Circle Pines, MN, 1984.
Bölte S, Poustka F . The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatry Hum Dev 2002; 33: 165–172.
Rose MD, Winston F, Hieter FP (eds). Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1990.
Koller HT, Klade T, Ellinger A, Breitenbach M . The yeast growth control gene GRC5 is highly homologues to the mammalian putative tumor suppressor gene QM. Yeast 1996; 12: 53–65.
Gietz RD, Sugino A . New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 1988; 74: 527–534.
Müller-Taubenberger A, Graack H-R, Grohmann L, Schleicher M, Gerisch G . An extended ubiquitin of Dictyostelium is located in the small ribosomal subunit. J Biol Chem 1989; 264: 5319–5322.
Cigan AM, Foiani M, Hannig AM, Hinnebusch AG . Complex formation by positive and negative translational regulators of GCN4. Mol Cell Biol 1991; 11: 3217–3228.
Nika J, Erickson FL, Hannig EM . Ribosomal protein L9 is the product of GRC5, a homolog of the putative tumor suppressor QM in S. cerevisiae. Yeast 1997; 13: 1155–1166.
Oender K, Loeffler M, Doppler E, Eder M, Lach S, Heinrich F et al. Translational regulator RpL10p/Grc5p interacts physically and functionally with Sed1p, a dynamic component of the yeast cell surface. Yeast 2003; 20: 281–294.
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA et al. (eds). Current protocols in molecular biology. John Wiley and Sons Inc.: NY, 1990.
Pachler K, Karl T, Kolmann K, Mehlmer N, Eder M, Loeffler M et al. Functional interaction in establishment of ribosomal integrity between small subunit protein rpS6 and translational regulator rpL10/Grc5p. FEMS Yeast Res 2004; 5: 271–280.
Dick FA, Trumpower BL . Heterologous complementation reveals that mutant alleles of QSR1 render 60S ribosomal subunits unstable and translationally inactive. Nucleic Acids Res 1998; 26: 2442–2448.
Brengues M, Teixeira D, Parker R . Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 2005; 310: 486–489.
Eisinger DP, Dick FA, Denke E, Trumpower BL . SQT1, which encodes an essential WD domain protein of Saccharomyces cerevisiae, suppresses dominant-negative mutations of the ribosomal protein gene QSR1. Mol Cell Biol 1997; 17: 5146–5155.
Draptchinskaia N, Gustavsson P, Andersson B, Petterson M, Willig T-N, Dianzani I et al. The gene encoding ribosomal protein S19 is mutated in Diamond–Blackfan anaemia. Nat Genet 1999; 21: 169–175.
Palmen SJM, van Engeland H, Hof PR, Schmitz C . Neuropathological findings in autism. Brain 2004; 127: 2572–2583.
Bauman ML, Kemper TL . The neuropathology of the autism spectrum disorders: what have we learned? Novartis Found Symp 2003; 251: 112–128.
McEwen BS . Possible mechanisms for atrophy of the human hippocampus. Mol Psychiatry 1997; 2: 255–262.
Johnston MV, Alemi L, Harum KH . Learning, memory and transcription factors. Pediatr Res 2003; 53: 369–374.
Jin P, Alisch RS, Warren ST . RNA and microRNAs in fragile X mental retardation. Nat Cell Biol 2004; 6: 1048–1053.
Bear MF, Huber KM, Warren ST . The mGluR theory of fragile X mental retardation. Trends Neurosci 2004; 27: 370–377.
Miyoshi K, Tsujii R, Yoshida H, Maki Y, Wada A, Matsui Y et al. Normal assembly of 60 S ribosomal subunits is required for the signaling in response to a secretory defect in Saccharomyces cerevisiae. J Biol Chem 2002; 277: 18334–18339.
Zhao Y, Sohn Y-H, Warner JR . Autoregulation in the biosynthesis of ribosomes. Mol Cell Biol 2004; 23: 699–707.
Just MA, Cherkassky VL, Keller TA, Minshew NJ . Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 2004; 127: 1811–1821.
Wickelgren I . Autistic brains out of synch? Science 2005; 308: 1856–1858.
Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 2003; 34: 27–29.
Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard M-P et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 2004; 74: 552–557.
Maestrini E, Lai C, Marlow A, Matthews N, Wallace S, Bailey A et al. Serotonin transporter (5-HTT) and γ-aminobutyric acid receptor subunit β3 (GABRB3) gene polymorphisms are not associated with autism in the IMGSA families. Am J Med Genet 1999; 88: 492–496.
Devlin B, Cook Jr EH, Coon H, Dawson G, Grigorenko EL, McMahon W et al. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry 2005; 10: 1110–1116.
Acknowledgements
We thank all the families for their cooperation and the professionals for their support in collecting data; S Bölte for establishment of the patient database; U Ernst, H Bausbacher and A Irsigler for excellent technical assistance; K Oender, M Loeffler and T Karl for expert advice on cloning strategies; H Zhu and J Zhou for expert assistance in ribosomal profile analyses; S Bechtel for stimulating discussions; S Wiemann and D Arlt for critical reading of the manuscript. LBK wishes to thank M Breitenbach for continuous and generous support of her work. This work was supported by grants from the Deutsche Forschungsgemeinschaft to AP and FP and in part by grant 01GR0420 of the National Genome Research Network, funded by the Bundesministerium für Bildung und Forschung, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klauck, S., Felder, B., Kolb-Kokocinski, A. et al. Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol Psychiatry 11, 1073–1084 (2006). https://doi.org/10.1038/sj.mp.4001883
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001883
Keywords
This article is cited by
-
A genome-wide methylation study reveals X chromosome and childhood trauma methylation alterations associated with borderline personality disorder
Translational Psychiatry (2021)
-
Neurobiological roots of psychopathy
Molecular Psychiatry (2020)
-
Mental health dished up—the use of iPSC models in neuropsychiatric research
Journal of Neural Transmission (2020)
-
Genome-wide mRNA expression analysis of peripheral blood from patients with obsessive-compulsive disorder
Scientific Reports (2018)
-
Heterogeneity and specialized functions of translation machinery: from genes to organisms
Nature Reviews Genetics (2018)