Abstract
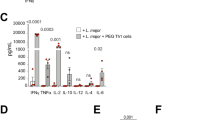
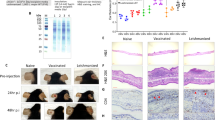
Severity of disease caused by Leishmania major depends on the genetics of the host. Early induction of T helper cell type 1 (Th1)-type responses in resistant C57BL/6 mice and T helper cell type 2 (Th2) in susceptible BALB/c mice is thought to determine cure or disease respectively. We have mapped three loci that confer susceptibility or resistance upon congenic mice on the C57BL/6 or BALB/c backgrounds. Here we examine the histopathology and production of interleukin 4 (IL-4) and interferon gamma (IFN-γ) in the skin and draining lymph nodes in the congenic and parental mice. We show an evolving granuloma with a staged infiltration of inflammatory cells, but no difference between the groups. As an indication of an early-polarised Th1/Th2 response we measured IFN-γ and IL-4 in the lymph nodes and found no difference between any of the mice during the first 48 h. During infection, the level of IL-4 correlated with the lesion size, indicating that IL-4 reflects the disease severity rather than controls it. Considering this effect, B6.C(lmr1,lmr2) mice had similar cytokine levels to the parental C57BL/6 mice despite increased susceptibility and C.B6(lmr1,lmr2) were similar to BALB/c despite increased resistance. We conclude that the lmr loci affect disease severity by a mechanism independent of conventional helper T-cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM . Reciprocal expression of interferon-g or interleukin-4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med 1989; 169: 59–72.
Himmelrich H, Launois P, Maillard I . In BALB/c mice IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol 2000; 164: 4819–4825.
Sacks D, Noben-Trauth N . The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2002; 2: 845–858.
Sadick MD, Street N, Mosmann TR, Locksley RM . Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect Immun 1991; 59: 4710–4714.
Mohrs M, Holscher C, Brombacher F . Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect Immun 2000; 68: 1773–1780.
Noben-Trauth N, Kropf P, Muller I . Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science 1996; 271: 987–990.
Blackwell JM . Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology 1996; 112: S67–S74.
Beebe AM, Mauze S, Schork NJ, Coffman RL . Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity 1997; 6: 551–557.
Roberts LJ, Baldwin TM, Curtis JM, Handman E, Foote SJ . Resistance to Leishmania major is linked to the H2 region on chromosome 17 and to chromosome 9. J Exp Med 1997; 185: 1–6.
Roberts LJ, Baldwin TM, Speed TP, Handman E, Foote SJ . Chromosomes X, 9, and the H2 locus interact epistatically to control Leishmania major infection. Eur J Immunol 1999; 29: 3047–3050.
Lipoldova M, Svobodova M, Krulova M . Susceptibility to Leishmania major infection in mice: multiple loci and heterogeneity of immunopathological phenotypes. Genes Immun 2000; 1: 200–206.
Elso CM, Roberts LJ, Smyth GK . Leishmaniasis host response loci (lmr1, 2 and 3) modify disease severity through a Th1/Th2 independent pathway. Genes Immun 2003, (in press).
Handman E, Hocking RE, Mitchell GF, Spithill TW . Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol 1983; 7: 111–126.
Mitchell GF, Curtis JM, Handman E, McKenzie IFC . Cutaneous leishmaniasis in mice: disease patterns in reconstituted nude mice of several genotypes infected with Leishmania tropica. Aust J Exp Biol Med Sci 1980; 58: 521–532.
Mitchell GF, Curtis JM, Handman E . Resistance to cutaneous leishmaniasis in genetically susceptible BALB/c mice. Aust J Exp Biol Med Sci 1981; 59: 555–565.
Simpson DA, Feeney S, Boyle C, Stitt AW . Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol Vis 2000; 6: 178–183.
Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA . In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1. Eur J Immunol 1995; 25: 3298–3307.
Abbas AK, Murphy KM, Sher A . Functional diversity of helper T cells. Nature 1996; 383: 787–793.
Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KJ . Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol 1987; 138: 744–749.
Morris L, Troutt AB, Handman E, Kelso A . Changes in the precursor frequencies of IL-4 and IFN-γ secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol 1992; 149: 2715–2721.
Baldwin T, Elso CM, Curtis J, Buckingham LA, Handman E . The site of Leishmania major infection determines the disease severity and immune responses. Infect Immun 2003; 71: 6830–6834.
Launois P, Maillard I, Pingel S . IL-4 rapidly produced by Vb4 Va8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 1997; 6: 541–549.
Biedermann T, Zimmermann S, Himmelrich H . IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol 2001; 2: 1054–1060.
Nabors GS, Farrell JP . Site-specific immunity to Leishmania major in SWR mice: the site of infection influences susceptibility and expression of the antileishmanial immune response. Infect Immun 1994; 62: 3655–3662.
Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM . Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity 2002; 17: 191–200.
Ritter U, Korner H . Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol 2002; 24: 295–301.
Shankar AH, Titus RG . T cell and non-T cell compartments can independently determine resistance to Leishmania major. J Exp Med 1995; 181: 845–855.
Tacchini-Cottier F, Zweifel C, Belkaid Y . An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 2000; 165: 2628–2636.
Aseffa A, Gumy A, Launois P . The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol 2002; 169: 3232–3241.
Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks D . CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420: 502–507.
Muraille E, De Trez C, Brait M . Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol 2003; 170: 4237–4241.
de Veer MJ, Curtis JM, Baldwin TM . MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and TLR2 signalling. Eur J Immunol 2003; 33: 2822–2831.
Matte C, Olivier M . Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. J Infect Dis 2002; 185: 673–681.
Aseffa A, Gummy A, Launois P . The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol 2002; 169: 3232–3241.
Belkaid Y, Mendez S, Lira R . A natural model of Leishmania major infection reveals a prolonged ‘silent’ phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol 2000; 165: 969–977.
Acknowledgements
This work was supported by the National Institutes of Health, USA and the National Health and Medical Research Council of Australia. Colleen Elso was supported by an Australian Postgraduate Award. We thank Joan Curtis and Lynn Buckingham for superb technical assistance and Jim Goding for the critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elso, C., Kumar, B., Smyth, G. et al. Dissociation of disease susceptibility, inflammation and cytokine profile in lmr1/2 congenic mice infected with Leishmania major. Genes Immun 5, 188–196 (2004). https://doi.org/10.1038/sj.gene.6364056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gene.6364056
Keywords
This article is cited by
-
Lessons from other diseases: granulomatous inflammation in leishmaniasis
Seminars in Immunopathology (2016)
-
A randomized controlled phase IIb wound healing trial of cutaneous leishmaniasis ulcers with 0.045% pharmaceutical chlorite (DAC N-055) with and without bipolar high frequency electro-cauterization versus intralesional antimony in Afghanistan
BMC Infectious Diseases (2014)