Abstract
Aim
To evaluate the effectiveness of anterior chemodenervation of levator palpebrae superioris with Botulinum toxin type A (Botox®) to induce temporary ptosis for corneal protection, and assess the incidence of superior rectus underaction.
Methods
Prospective interventional case series. Patients with ocular surface pathology requiring temporary tarsorrhaphy underwent transcutaneous anterior chemodenervation of levator palpebrae superioris with Botox®. The onset and duration of ptosis, corneal healing, and superior rectus underaction was evaluated.
Results
Ten eyes of 10 patients underwent transcutaneous anterior chemodenervation of levator muscle. Five patients had Bells palsy with exposure keratopathy, four patients had persistent epithelial defect, and one had neurotrophic ulcer. The median age at presentation was 30 years. Median dose of Botulinum toxin injection was 12.5 U (range 10–15 U). The mean palpebral fissure height of 9 mm (SD±2.1 mm) before injection, reduced to 2.8 mm (SD±1.9 mm) at 1-week post-injection. More than 50% reduction in palpebral fissure height was seen in nine out of 10 eyes (90%, 95% CI 71.4–100%) at 1 week, seven of nine eyes (77.8%, 95% CI 50.6–100%) at 2 weeks, and two of nine eyes (22.2%, 95% CI 0–49.4%) at 4 weeks, and returned to pretreatment level after mean duration of 9.2 weeks (range 5–16 weeks). Superior rectus underaction was not noted in any of the patient (95% CI 0–30%). Corneal pathology improved in all cases.
Conclusion
Anterior chemodenervation of levator palpebrae superioris with Botulinum toxin type A (Botox®) induces significant temporary ptosis and aids in corneal healing. Anterior placement of the toxin injection may avoid superior rectus underaction.
Similar content being viewed by others
Introduction
Temporary protection of the cornea is desirable in patients with exposure keratopathy owing to Bell's palsy, persistent epithelial defects, and indolent corneal ulcers. Tarsorrhaphy has remained the traditional treatment for temporary protection of the cornea. It however requires a surgical procedure, can lead to scarring of the eyelid margin, and is cosmetically disfiguring. Moreover, it makes the evaluation of the ocular surface difficult.
Botulinum toxin injection into the levator palpebrae superioris has been reported to be an effective method to induce temporary ptosis for corneal protection.1, 2, 3, 4, 5, 6 Transient superior rectus underaction is the most commonly reported side effect, seen in 68–80% of treated patients.1, 2 Adams et al in their series noted that 12 out of 15 (80%) patients developed superior rectus underaction, of which 10 (67%) continued to demonstrate underaction even after complete resolution of ptosis.1 Kirkness et al noted a 68% incidence of superior rectus underaction along with ptosis induction.2 Although the mean recovery time of superior rectus underaction was noted to be 6 weeks, four (16%) patients in their series had diplopia lasting beyond complete recovery of ptosis.2
Protective ptosis accompanied by superior rectus underaction has several disadvantages. The reduced Bells reflex and the resultant hypotropia can lead to a paradoxical increased exposure of the cornea through the reduced palpebral fissure. In patients with good visual acuity (Bells palsy) in the affected eye, the induced protective ptosis is often incomplete, and superior rectus underaction in these patients can lead to significant diplopia, especially as the ptosis recovers. Following chemodenervation of levator muscle, persisting hypotropias owing to breakdown of fusion or contracture of ipsilateral inferior rectus muscle requiring corrective strabismus surgery has also been reported.7
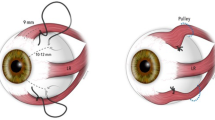
A technique of levator chemodenervation with reduced chances of superior rectus underaction would therefore be highly desirable. Prior reports have described administration of Botulinum toxin with a 25 mm needle aiming for an orbital injection into the belly of the levator muscle.1, 2, 3, 7 It is possible that the anatomic proximity of the levator and superior rectus muscle within the mid-orbit could have led to the high incidence of superior rectus underaction following posterior placement of the Botulinum toxin.1, 2, 3, 8 The risk of inadvertent intraocular injection of the toxin also increases with deep injections.9, 10
In this series, we report our experience with anterior chemodenervation of levator palpebrae superioris for induction of temporary ptosis, and its effectiveness in preventing superior rectus underaction. The advantages of this technique are discussed.
Materials and methods
This prospective interventional study was performed at the Ophthalmic plastic surgery service of LV Prasad Eye Institute, Hyderabad, India between March 2003 and February 2004. Only patients who met the eligibility criteria during the study period, for whom leftover Botox® (within 24 h of reconstitution) was available, were included in this study. All patients were referred by our cornea services requesting trasorrhaphy for corneal protection. The demographic data of the patient, corneal pathology, pre-injection palpebral fissure height, ocular motility, and lagophthalmos were measured. The study complied with the policies of the institutional review board of LV Prasad Eye Institute, Hyderabad.
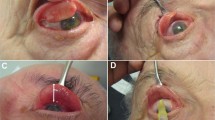
Transcutaneous chemodenervation of levator palpebrae superioris was performed using Botulinum Toxin type A (Botox®, Allergan, Irvine CA, USA) reconstituted with 2cc preservative free normal saline (Figure 1). A tuberculin syringe with a half-inch 26- or 30-gauge needle was used for injection procedure. The needle tip was placed near the anterior orbital roof just behind the superior orbital rim in the mid-pupillary plane, and the toxin was injected. The patient was continued on appropriate topical medications as prescribed by the corneal surgeon.
A 10-year-old female with right meta-herpetic corneal ulcer (top left). Anterior transcutaneous chemodenervation of levator muscle was performed using 10 U of Botox® with a half-inch 26-gauge needle (top right). One week after chemodenervation demonstrating complete ptosis (bottom left). Patient in upgaze demonstrating preserved superior rectus function (bottom right). (Reprinted with permission, Indian Journal of Ophthalmology, December 2005).
The extent of exposure keratopathy and corneal pathology was monitored by the corneal surgeon with fluorescein staining or measurement of epithelial defect at each visit. The palpebral fissure height, lagophthalmos, corneal pathology, and ocular motility were evaluated at 1, 2, and 4 weeks after chemodenervation. Superior rectus underaction was graded as Grade 0 (no underaction) to Grade 4 (complete hypotropia with no supraduction). The mean difference in palpebral fissure height was compared by Wilcoxon signed rank test, and the incidence of superior rectus underaction was evaluated.
Results
Ten eyes of 10 patients underwent transcutaneous anterior chemodenervation of levator muscle during the 1-year study period. Five patients had Bells palsy with exposure keratopathy within 1 month of onset, four patients had persistent epithelial defect following penetrating keratoplasty, and one patient had neurotrophic keratopathy. The median age at presentation was 30 years (range 4–57 years).
The median dose of Botulinum toxin (Botox®) injection was 12.5 U (range 10–15 U). The mean palpebral fissure height was 9 mm (SD±2.1 mm) before injection, and reduced to 2.8 mm (SD±1.9 mm) at 1 week post-injection. The mean difference in the palpebral fissure height was 6.2 mm (95% CI, 4.9–7.4 mm), and was statistically significant (P=0.005; Wilcoxon signed rank test). The mean percentage reduction in palpebral fissure height was 70.4% (SD±17.4). Nine patients had more than 1-week follow-up post-chemodenervation. More than 50% reduction in palpebral fissure height was seen in nine out of 10 eyes (90%, 95% CI 71.4–100%) at 1 week, seven of nine eyes (77.8%, 95% CI 50.6–100%) at 2 weeks, and two of nine eyes (22.2%, 95% CI 0–49.4%) at 4 weeks post-injection (Table 1). The palpebral fissure height returned to pretreatment level after a mean duration of 9.2 weeks (range 5–16 weeks). Corneal pathology improved in all cases.
Superior rectus underaction was not noted in any patient during the assessment made at 1, 2, and 4 weeks during the study period. No patient reported diplopia during or after complete recovery of induced ptosis.
The comparison of toxin dose, mean duration of action, and superior rectus underaction in various reported series is summarized in Table 2.
Discussion
Several reports of Botulinum toxin injection into the levator palpebrae superioris for corneal protection have been published.1, 2, 3, 4, 5, 6 Adams et al reported induction of ptosis with botulinum toxin that lasted for a mean duration of 8.1 weeks.1 Temporary underaction of the superior rectus muscle was noted in 12 out of 15 (80%) cases in their series, and 10 patients (67%) had persistence of superior rectus underaction beyond complete recovery of ptosis. Two patients in their series noted diplopia upon recovery of ptosis, and one patient required prisms for 3 months.
Kirkness et al later reported a series of 25 cases where protective ptosis was induced with Botulinum toxin type A in a similar manner.2 The authors reported complete ptosis in 75% cases lasting for an average of 16 days and recovery within 8.5 weeks. Temporary underaction of the superior rectus muscle was noted in 17 out of 25 (68%) cases, recovering within 6 weeks. Four (16%) patients in their series had diplopia lasting beyond complete recovery of ptosis.2 In both the studies, Dysport (Vaccine research and Production Laboratory, Porton Down) was used in a concentration of 0.0625 ng.
Ellis and Daniell recently reported a series of 21 cases in which the average duration of induced ptosis was 46 days.3 Diplopia was noted in five (24%) cases in their series, but the exact duration of superior rectus underaction was not mentioned. Moreover, the effective dose of Botulinum toxin (Botox®) was 5 U, with one-third patients requiring repeat injections to maintain adequate ptosis. Heyworth and Lee reported persisting hypotropia following induction of protective ptosis for corneal protection that required strabismus surgery.7 The proposed mechanism was either breakdown of fusion owing to prolonged occlusion, or contracture of ipsilateral antagonist muscle. A pre-existing latent vertical deviation was considered as a risk factor for development of persisting hypotropia.7
Superior rectus underaction following induction of ptosis can reduce the Bells reflex. The resultant hypotropia is likely to further expose the healing cornea, especially while the palpebral fissure gradually widens with ptosis recovery. This in turn can negate the effects of the induced protective ptosis. It would therefore be desirable to induce protective ptosis without causing underaction of superior rectus muscle to avoid its attendant complications.
Prior reports have described administration of Botulinum toxin with a 25 mm needle aiming for an orbital injection into the belly of levator muscle.1, 2, 3, 7 It is possible that the posterior placement of Botulinum toxin could have led to the high incidence of superior rectus underaction. The levator muscle belly and the superior rectus are in close approximation behind the Whitnall's ligament. Moreover, the lateral border of superior rectus muscle extends beyond that of the levator muscle in the mid and posterior third of the orbit.8 This anatomic relation may cause the toxin to act on the superior rectus muscle when injected deep into the mid-orbit. Several incidences of globe perforation have been reported with Botulinum toxin injection for strabismus.9, 10 The use of a long needle and posterior injection increases the possibility of globe perforation.
In our study, the injection was aimed at the anterior most part of the levator muscle, using a half-inch needle. The induced ptosis that lasted for a mean duration of 9.2 weeks in our series is more than previous reports (Table 2), probably owing to the higher toxin dose used by us (10–15 U of Botox®). Since a higher initial dose was used, we did not require repeat injections to maintain ptosis as reported by Ellis and Daniell.3 Surprisingly, despite the use of a comparatively higher dose in our series (Table 2), the anterior placement of the toxin prevented superior rectus underaction.
The anterior placement of Botulinum toxin injection therefore has several advantages. It avoids associated superior rectus underaction, which was previously considered inevitable and self-resolving. Anterior placement of toxin allows a higher initial dose, thereby avoiding repeat injections, and maintains a longer duration of induced ptosis. The use of a half-inch needle may also potentially reduce the chances of globe perforation.
It can be argued that the superior rectus underaction in previous reports was temporary, and is unlikely to induce diplopia, while the eyelid is ptotic. However, it is important to note that induced ptosis is often incomplete (Bells palsy), especially as the ptosis recovers. In these cases, hypotropia during initial few weeks can cause diplopia or compromise corneal healing.
Our study comprises of a small sample size, and is non-comparative. Moreover, the difference in bio-equivalence between Botox® and Dysport® makes comparison with previous studies difficult. However, this prospective series suggests that anterior chemodenervation of the levator is likely to reduce the chances of simultaneous superior rectus underaction. A comparative study randomizing both techniques of injection using standard toxin dose would be required to further assess its validity.
References
Adams GG, Kirkness CM, Lee JP . Botulinum toxin A induced protective ptosis. Eye 1987; 1 (Part 5): 603–608.
Kirkness CM, Adams GG, Dilly PN, Lee JP . Botulinum toxin A-induced protective ptosis in corneal disease. Ophthalmology 1988; 95 (4): 473–480.
Ellis MF, Daniell M . An evaluation of the safety and efficacy of botulinum toxin type A (BOTOX) when used to produce a protective ptosis. Clin Exp Ophthalmol 2001; 29 (6): 394–399.
Wuebbolt GE, Drummond G . Temporary tarsorrhaphy induced with type A Botulinum toxin. Can J Ophthalmol 1991; 26: 383–385.
Smyth AG . Protective ptosis after parotid surgery induced with Botulinum toxin. Br J Oral Maxillofac Surg 1995; 33: 107–109.
Magoon EH . Botulinum injection for treatment of blepharospasm, corneal exposure and entropion. J Ocul Ther Surg 1985; 4: 133–135.
Heyworth PL, Lee JP . Persisting hypotropias following protective ptosis induced by botulinum neurotoxin. Eye 1994; 8 (Part 5): 511–515.
Bron AJ, Tripathi RC, Tripathi BJ . The extraocular muscles and ocular movements. In: Bron AJ, Tripathi RC, Tripathi BJ (eds). Wolff's Anatomy of the Eye and Orbit, 8th edn. Chapman & Hall: London, 1997, pp 147–152.
Scott AB . Botulinum toxin treatment of strabismus. Focal points 1989: Clinical Modules for Ophthalmologists, Vol 7, Module 12. American Academy of Ophthalmology: San Francisco, 1989, pp 1–11.
Liu M, Lee HC, Hertle RW, Ho AC . Retinal detachment from inadvertent intraocular injection of botulinum toxin A. Am J Ophthalmol 2004; 137 (1): 201–202.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 23rd Annual Meeting of the European Society of Ophthalmic plastics and Reconstructive Surgery, Greece, 15–17 September, 2005
Authors do not have any financial interest in the results presented. This research was funded by Hyderabad Eye Research Foundation, Hyderabad, India.
Rights and permissions
About this article
Cite this article
Naik, M., Gangopadhyay, N., Fernandes, M. et al. Anterior chemodenervation of levator palpebrae superioris with botulinum toxin type-A (Botox®) to induce temporary ptosis for corneal protection. Eye 22, 1132–1136 (2008). https://doi.org/10.1038/sj.eye.6702866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702866
Keywords
This article is cited by
-
A modified transconjunctival technique for botulinum toxin chemodenervation of levator palpebrae superioris for corneal protection
Eye (2022)
-
Adverse Events Associated With Botox as Reported in a Food and Drug Administration Database
Aesthetic Plastic Surgery (2021)
-
Paralytic Lagophthalmos: Comprehensive Approach to Management
Current Otorhinolaryngology Reports (2018)
-
Corneal Transplantation in the Setting of Neurotrophic Keratopathy—Risks and Considerations
Current Ophthalmology Reports (2017)
-
Botulinumtoxin bei okulären Folgen nach Fazialisparese
Der Ophthalmologe (2012)