Summary:
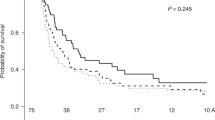
We retrospectively reviewed the results of transplanting peripheral blood progenitor cell (PBPC) allografts from HLA-matched sibling donors mobilized using various hematopoietic cytokines. Patients had received allografts mobilized with Granulocyte colony-stimulating factor (G-CSF) (G, N=65) alone, G plus Granulocyte-macrophage colony stimulating factor (GM-CSF) (G/GM, N=70), or GM-CSF alone at 10 or 15 μg/kg/day (GM, N=10 at 10 μg/kg/day and 21 at 15 μg/kg/day). The CD34+ and CD3+ cell content of grafts were significantly lower following GM alone compared to G alone (P<0.001 and 0.04, respectively). Nonhematopoietic toxicity observed in donors precluded dose escalation of GM-CSF beyond 10 μg/kg/day. Hematopoietic recovery was similar among all three groups. Grades II–IV acute graft-versus-host disease (GVHD) was observed in only 13% of patients in the GM alone group compared to 49 and 69% in the G alone or G/GM groups, respectively (P<0.001). In a multivariate analysis, receipt of PBPC mobilized with GM alone was associated with a lower risk of grades II–IV acute GVHD (hazard ratio 0.21; 95% CI 0.073, 0.58) compared to G alone or G/GM. There were no differences in relapse risk or overall survival among the groups. Donor PBPC grafts mobilized with GM-CSF alone result in prompt hematopoietic engraftment despite lower CD34+ cell doses and may reduce the risk of grades II–IV acute GVHD following HLA-matched PBPC transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blaise D, Kuentz M, Fortanier C et al. Randomized trial of bone marrow vs lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol 2000; 18: 537–546.
Heldal D, Tjonnfjord G, Brinch L et al. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant 2000; 25: 1129–1136.
Vigorito AC, Azevedo WM, Marques JF et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant 1998; 22: 1145–1151.
Couban S, Simpson DR, Barnett MJ . A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100: 1525–1531.
Powles R, Mehta J, Kulkarni S et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet 2000; 355: 1231–1237.
Schmitz N, Beksac M, Hasenclever D et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood 2002; 100: 761–767.
Bensinger WI, Martin PJ, Storer B et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001; 344: 175–181.
Korbling M, Anderlini P . Peripheral blood stem cell vs bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood 2001; 98: 2900–2908.
Cutler C, Giri S, Jeyapalan S et al. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol 2001; 19: 3685–3691.
Mohty M, Bilger K, Jourdan E et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia 2003; 17: 869–875.
Schrezenmeier H, Bredeson C, Bruno B et al. Comparison of allogeneic bone marrow and peripheral blood stem cell transplantation for aplastic anemia: collaborative study of european blood and marrow transplant group and international bone marrow transplant registry. Blood 2003; 102: 79a (abstract).
Eapen M, Horowitz MM, Klein JP et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: The Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol 2004; 22: 4872–4880.
Spitzer G, Adkins D, Mathews M et al. Randomized comparison of G-CSF + GM-CSF vs G-CSF alone for mobilization of peripheral blood stem cells: effects on hematopoietic recovery after high-dose chemotherapy. Bone Marrow Transplant 1997; 20: 921–930.
To LB, Haylock DN, Simmons PJ, Juttner CA . The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Socinski MA, Cannistra SA, Elias A et al. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet 1988; 1: 1194–1198.
Fischmeister G, Kurz M, Haas OA et al. G-CSF vs GM-CSF for stimulation of peripheral blood progenitor cells (PBPC) and leukocytes in healthy volunteers: comparison of efficacy and tolerability. Ann Hematol 1999; 78: 117–123.
Sohn SK, Kim JG, Seo KW et al. GM-CSF-based mobilization effect in normal healthy donors for allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2002; 30: 81–86.
Lane TA, Ho AD, Bashey A et al. Mobilization of blood-derived stem and progenitor cells in normal subjects by granulocyte-macrophage- and granulocyte-colony-stimulating factors. Transfusion 1999; 39: 39–47.
Gazitt Y . Comparison between granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the mobilization of peripheral blood stem cells. Curr Opin Hematol 2002; 9: 190–198.
Devine S, Adkins D, Khoury H et al. Mobilization of donors with GM-CSF plus G-CSF or GM-CSF alone results in significantly different graft composition compared to G-CSF alone. Blood 2002; 100: 825a (abstract).
Blum W, Brown R, Lin HS et al. Low-dose (550 cGy), single-exposure total body irradiation and cyclophosphamide: consistent, durable engraftment of related-donor peripheral blood stem cells with low treatment-related mortality and fatal organ toxicity. Biol Blood Marrow Transplant 2002; 8: 608–618.
Hughes WT, Armstrong D, Bodey GP et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34: 730–751.
Shulman HM, Sullivan KM, Weiden PL et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Roscoe RA, Rybka WB, Winkelstein A et al. Enumeration of CD34+ hematopoietic stem cells for reconstitution following myeloablative therapy. Cytometry 1994; 16: 74–79.
Heimfeld S . HLA-identical stem cell transplantation: is there an optimal CD34 cell dose? Bone Marrow Transplant 2003; 31: 839–845.
Heimfeld S . Bone marrow transplantation: how important is CD34 cell dose in HLA-identical stem cell transplantation? Leukemia 2003; 17: 856–858.
Urbano-Ispizua A . High stem cell dose in haemopoietic transplantation: is it always beneficial? Leukemia 2003; 17: 1467–1469.
Vasu C, Dogan R-NE, Holterman MJ, Prabhakar BS . Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol 2003; 170: 5511–5522.
Miller G, Pillarisetty VG, Shah AB et al. Endogenous granulocyte-macrophage colony-stimulating factor overexpression in vivo results in the long-term recruitment of a distinct dendritic cell population with enhanced immunostimulatory function. J Immunol 2002; 169: 2875–2885.
Parajuli P, Mosley RL, Pisarev V et al. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp Hematol 2001; 29: 1185–1193.
Taylor PA, Lees CJ, Blazar BR . The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002; 99: 3493–3499.
Edinger M, Hoffmann P, Ermann J et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150.
O'Keeffe M, Hochrein H, Vremec D et al. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood 2002; 99: 2122–2130.
Daro E, Butz E, Smith J et al. comparison of the functional properties of murine dendritic cells generated in vivo with Flt3 ligand, GM-CSF and flt3 ligand plus GM-CSF. Cytokine 2002; 17: 119–130.
Vogelsang GB . How I treat chronic graft-versus-host disease. Blood 2001; 97: 1196–1201.
Bhushan V, Collins Jr RH . Chronic graft-vs-host disease. JAMA 2003; 290: 2599–2603.
Przepiorka D, Anderlini P, Saliba R et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 2001; 98: 1695–1700.
Zaucha JM, Gooley T, Bensinger WI et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood 2001; 98: 3221–3227.
Lane TA, Law P, Maruyama M et al. Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood 1995; 85: 275–282.
Ho AD, Young D, Maruyama M et al. Pluripotent and lineage-committed CD34+ subsets in leukapheresis products mobilized by G-CSF, GM-CSF vs a combination of both. Exp Hematol 1996; 24: 1460–1468.
Przepiorka D, Smith TL, Folloder J et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 1999; 94: 1465–1470.
Thomson KJ, Ings S, Watts M et al. CD34+ cell dose and the occurrence of GVHD in the presence of in vivo T-cell depletion. Blood 2004; 103: 743.
Acknowledgements
This study was supported (in part) by research funding from Berlex Inc. to SMD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devine, S., Brown, R., Mathews, V. et al. Reduced risk of acute GVHD following mobilization of HLA-identical sibling donors with GM-CSF alone. Bone Marrow Transplant 36, 531–538 (2005). https://doi.org/10.1038/sj.bmt.1705091
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705091
Keywords
This article is cited by
-
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials
Stem Cell Research & Therapy (2022)
-
Novel agents and approaches for stem cell mobilization in normal donors and patients
Bone Marrow Transplantation (2012)
-
Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF?
Bone Marrow Transplantation (2007)