Abstract
Septins are an evolutionarily conserved family of genes that encode a P loop-based GTP-binding domain flanked by a polybasic domain and (usually) a coiled-coil region. They have roles in cytokinesis, vesicle trafficking, polarity determination, and can form membrane diffusion barriers, as well as in microtubule and actin dynamics. Septins can form hetero-oligomeric complexes and possibly function as dynamic protein scaffolds. Recently, it has been shown that there are at least 13 human septin genes that exhibit extensive alternate splicing. There are complex patterns of human septin gene expression and recently it has been found that alterations in septin expression are seen in human diseases including neoplasia. This review summarises the essential properties of septins and outlines the accumulating evidence for their involvement in human neoplasia. Septins may belong to the class of cancer critical genes where alteration in expression profile (including alterations in the spectrum of transcripts expressed) may underpin their role in neoplasia as opposed to specific mutational events.
Similar content being viewed by others
Main
In 1973, a group of cytokinesis mutants were identified in budding yeast by Hartwell. The protein products of these genes (ScCdc3, ScCdc10, ScCdc11 and ScCdc12) localise to filamentous structures in the bud neck in Saccharomyces cerevisiae and were named septins by the laboratory of John Pringle. Much progress has been made in defining the range of functions of yeast septins and in the past 5 years their role in disease states, including cancer, has become apparent (Hall and Russell, 2004). Septins appear to function in yeast as spatial landmarks, elements of the polarity determination apparatus and diffusion barriers (reviewed in Irazoqui and Lew, 2004; Finger, 2005). They all have a central P loop-based GTP-binding domain and there is evidence that septins form hetero- and homo-oligomeric structures and can form filaments, although their significance remains uncertain (Hall and Russell, 2004). However, post translational modifications such as phosphorylation (Dobbelaere et al, 2003) and protein–protein interactions (Casamayor and Snyder, 2003) are crucial to septin function. They have been suggested to form scaffold-like structures upon which other proteins bind, thus allowing proper spatial and temporal control of processes such as polarity determination and cytokinesis. The septins are evolutionarily conserved although, interestingly, septin-like sequences have not been identified in plants to date, and cannot be found in Dictyostelium. Furthermore, it is curious that the number of septin genes differs in phylogeny, with seven in yeast, five in Drosophila and only two in Caenorhabditis elegans (Hall and Russell, 2004). Considerable expansion of the number of septin genes is seen in vertebrates and 13 are now known in man (Hall et al, 2005). These septin genes are distributed widely in the human genome as might be expected from an evolutionarily ancient gene family, but there remains considerable sequence conservation as well as an extraordinary conservation of certain aspects of genomic architecture and gene control.
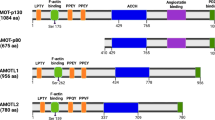
The nomenclature of mammalian septins has proven problematic but a uniform nomenclature has simplified a Babel-like array of terms (see Table 1 in Hall and Russell (2004) for the diverse septin aliases). The 13 known human septins have remarkable similarity, all having in their longest forms (see Figure 1) a central GTP-binding domain flanked by a polybasic region and a so-called septin unique domain (Versele and Thorner, 2005). The function of the GTP-binding domain remains controversial. Although there are similarities with the small rho-like GTPases, it is not clear that the GTP- or GDP-bound state of septin has true signalling properties. It may be that like other GTP-binding proteins, such as tubulin, the GTP (or GDP) status confers structural properties and may influence oligomerisation. The polybasic domain of SEPT4 has been shown to bind phosphoinositol phosphates, and a reciprocal relationship between GTP and PIP2 binding has been reported (Casamayor and Snyder, 2003). It may be that targeting of septins to membrane domains is relevant to some of their functions including the potential to act as a diffusion barrier in both yeast and mammalian cells (reviewed in Finger, 2005). Most, but not all, septins have C-terminal coiled-coil domains that fall into two groups by amino-acid sequence SEPT6, 8, 10, 11 and SEPT1, 2, 4, 5, 7 and 13 (Hall et al, 2005). In contrast, SEPT3, 9 and 12 have a shorter C terminus without a coiled coil. This recapitulates the budding yeast septins where ScCdc10 has no coiled coil whereas the others do. Three of the human septins (SEPT4, 8 and 9) have long N-terminal extensions, which have regions rich in proline residues.
The human septins. The longest known versions of the 13 human septins described to date including their chromosomal location. Four human septins can form fusion proteins with the N-terminal moiety of MLL (arrowed). All have a polybasic domain (PB), although some are less basic (PB with lighter shade), a GTP-binding domain (GBD) and a septin unique domain (SUD). Some have a coiled-coil domain at the C terminus (CC). The longest forms of SEPT4, 8 and 9 have long N-terminal extensions with regions rich in prolines (PRD).
The complexity of this gene family is increased by the existence of alternate splicing in most human septins, which dramatically increases the number of potential isoforms expressed. In the most extreme case so far defined, SEPT9, six 5′ splice variants can combine a common core domain with three 3′ splice variants to give at least 18 transcripts (McIlhatton et al, 2001) encoding 15 polypeptides. The discrepancy between the number of transcripts and isoforms is explained by the existence of two different 5′ transcripts that encode the same polypeptide. Many of the known isoforms encode truncated versions of a particular septin, which may act as (regulatory) dominant negative forms whose levels might modulate complex formation. In addition, the extraordinary observation of multiple splice variants encoding the same polypeptide has also been reported for SEPT8 and SEPT6. The genomic, transcriptional and isoform complexity, coupled with the sheer number of humans septins, has hindered progress in our understanding of this family of genes but some progress has recently been made.
Comprehensive expression profiling of all members of the human septin family indicates that some septins are expressed in all tissues (eg SEPT9), while others have restricted profiles, with, for example, SEPT3 only being found in the brain (Hall et al, 2005). Alterations in septin expression are seen in cancer and in other disease states. However, despite the size of the data sets studied, the complexity of splicing indicates that this global analysis can only be viewed as a first approximation to the definition of septin expression in man. In particular, the current data are inadequate for the delineation of the potential array of septin hetero-oligomers that might exist. Proteomic and biochemical analyses of specific septins suggest that some specific complexes can form (Versele and Thorner, 2005) and that, for example, SEPT2, 6 and 7 can form a stoichiometric association. In addition, specific coregulation can occur and experimental knock down of one component of this complex by siRNA leads to loss of expression of the other proteins in this complex. Furthermore, in mice lacking SEPT5, there is compensatory alteration in other septins (reviewed in Hall and Russell, 2004). Such data indicate that understanding the nature of septin complexes and their regulation are a central issue in the field.
A further crucial issue is the definition of the functional properties of human septins and an understanding of their biochemical attributes. While originally identified as a consequence of cytokinesis defects, it is clear that even in yeast, septins seem to have multiple functions (Hall and Russell, 2004). It would not be surprising if the increased number and complex distribution of mammalian septins is associated with an increased range of cellular functions. Data from multiple sources indicate that human septins can interact with other septins, as well as with components of the cytoskeleton such as actin and tubulin. In addition, interactions with S100A4, BORG3 and components of the exocytosis pathway have been reported (reviewed in Hall and Russell, 2004) and one truncated SEPT4 isoform has been associated with the induction of apoptosis via an interaction with XIAP (see below). Finally, recent data suggest a link with the small rho GTPases since the N terminus of SEPT9 binds a rhoGEF (Nagata and Inagaki, 2005).
Septins and cancer
Septins: the MLL connection
The first clues to the role of septins in neoplasia came from the observation that balanced translocations involving septin loci and the MLL locus on chromosome 11 were seen in leukaemia giving rise to chimeric fusion proteins where the N terminus of MLL was fused, in frame, to almost the entire open reading frame of SEPT9 (Osaka et al, 1999). Subsequently, it has been found that three other septins (SEPT5, SEPT6 and SEPT11) can form very similar fusion proteins with MLL again with the N-terminal moiety of MLL fused to almost the entire open reading frame of the partner septin (Taki et al, 1999; Ono et al, 2002; Kojima et al, 2004). MLL is a remarkably promiscuous gene, forming in-frame chimeras with more than 50 other genes. Current data suggest that these fusion partners fall into two distinct groups: those with a potent transactivation domain and those that possess potential oligomerisation motifs. The septins do not possess an activation domain but are believed to oligomerise, possibly via their coiled-coil domains. However, SEPT9 does not contain a C-terminal coiled coil and one must thus posit a role for an alternative domain in forming oligomers. Some recent data support the idea that oligomerisation by the septin moiety of MLL fusions is important (Ono et al, 2005) and also points to a possible role for the GTP-binding domain in the formation of dimers.
Linking SEPT9 and cancer
SEPT9 was linked to neoplasia by two other observations. Sorensen et al (2000) identified the murine SEPT9 locus as a common integration site for the SL-3 retrovirus in T-cell lymphomas. Thus, insertional mutagenesis at this locus suggests that SEPT9 can contribute to neoplasia. Independently, the human SEPT9 locus at 17q25.3 was identified as a common site for allelic imbalance in sporadic ovarian (Russell et al, 2000) and breast cancer (Kalikin et al, 2000). While mutations have not been observed in the known open reading frames of SEPT9, there is now abundant evidence pointing to altered expression of SEPT9 in ovarian (Burrows et al, 2003) and breast (Montagna et al, 2003) tumours. Indeed SEPT9 overexpression has been observed in diverse tumour types (Scott et al, 2005a). Of note is the observation that neoplasia is associated not just with altered expression of SEPT9 but also by alterations in the expression of specific SEPT9 transcripts with the SEPT9_v4 transcript being predominant in normal tissues but being replaced by SEPT9_v4* in tumours (Burrows et al, 2003; Scott et al, 2005b). These transcripts encode the same polypeptide but differ in their 5′ UTR sequences. The SEPT9_v4* transcript appears to be translated more efficiently than the SEPT9_v4 transcript (Russell and McDade, unpublished), and thus this change in transcript profile has a profound effect on the level of this SEPT9_v4 protein isoform.
Other septins and cancer
Several lines of evidence have suggested that SEPT4 may be involved in neoplasia. Tanaka et al (2002) identified two alternate splice variants of SEPT4 (and named it Bradeion) by screening an expression library. While expression of these transcripts is generally restricted to the brain, in tumours ectopic expression is observed. Furthermore, ribozyme-mediated downregulation of these transcripts could inhibit growth and tumorigenesis of colorectal cancer in vivo and in vitro and might be a useful diagnostic target (Tanaka et al, 2002). Independently, Larisch et al (2000) observed that what is now known as a SEPT4 transcript (and was previously named ARTS) could promote TGF beta-mediated apoptosis. It has been reported that the SEPT4 isoform encoded by this transcript binds to and can modulate the function of XIAP and thus promote apoptosis (Gottfried et al, 2004). Subsequent studies have suggested that the expression of this transcript (which is distinct from those reported by Tanaka et al) might function as a tumour suppressor since expression is lost in most cases of childhood ALL (Elhasid et al, 2004). As if the terminological morass of SEPT4 (which has been named H5, bradeion, Pnutl2, ARTS, MAART, hCDCrel-2 and Septin-M) were not enough, the situation has become more complex with the report of SEPT4 knockout mice, which have not yet been reported to be tumour prone (Ihara et al, 2005). The apparent absence of a tumour phenotype may reflect the nature of the gene-targeting events (in effect deleting the entire locus) rather than excluding a role for SEPT4 in neoplasia. Indeed, it again underscores the need for transcript-specific analysis of septins, as is the case for SEPT9.
Other septins have been linked to neoplasia and the first human septin to be systematically studied, SEPT2 (previously known as Nedd5), was shown to be required for cytokinesis and to bind actin and associate with focal adhesions. Recent data suggest that SEPT2 can have a role in chromosome congression and segregation and that altered expression of SEPT2 might promote abnormalities of these crucial processes, leading to disordered chromosomal dynamics, and underlie the development of aneuploidy (Spiliotis et al, 2005). Whether these data are relevant to human tumours is as yet uncertain but certainly deserves further study. Our current catalogue of septin expression changes seen in neoplasia (and other disease states) remains far from complete and the complex splicing events seen in the septins makes progress difficult. Nevertheless, the available data suggest that at least some septins can be implicated in human (and murine) neoplasia. The crucial question is how?
How do septins contribute to neoplasia?
The role of septins in cytokinesis would lead to the notion that these proteins are involved in neoplasia by perturbing cell division in some way. While this idea deserves attention and is supported by the recent observations of Spiliotis et al (2005), other possible explanations for the role of septins in neoplasia might be considered. The observation that septins can be involved in membrane dynamics is of interest given the increasingly recognised role of enhanced membrane dynamics in cancer (Polo et al, 2004). Another tantalising observation is the suggestion that one isoform of SEPT4 (previously called ARTS) can promote apoptosis (discussed above). Loss of function of this isoform might then reduce apoptosis and promote increase in cell number. Another observation of relevance to a potential role in neoplasia is the association of septins with both the actin and tubulin cytoskeleton (Surka et al, 2002; Nagata et al, 2003). Recently, Chacko et al (2005) have shown that the increased expression of the SEPT9_v4 protein has potent effects on the phenotype of epithelial cells. This isoform induces marked morphological changes in cultured cells with the generation of dramatic actin reorganisation and the formation of actin-based projections. In addition, SEPT9_v4 expression promotes cell motility in both two- and three-dimensional assays, and expression of GV mutants of SEPT9_v4 (analogous to gain of function mutants in GTP binding such as G12V in ras) promotes motility and perturbs the directionality of movement. These latter data are complemented by Golgi reorientation assays that suggest that SEPT9_v4 can alter cell polarity. This is perhaps not surprising given the role of septins in determining polarity in yeast (Irazoqui and Lew, 2004) and Finger et al (2003) have shown that a nematode septin can profoundly influence directional movement of developing neurons. How SEPT9_v4 induces these phenotypes remains uncertain but it is of note that the normal association of other SEPT9 isoforms with filamentous structures is perturbed by SEPT9_v4 and the GV mutant thereof (Chacko et al, 2005). SEPT9_v4 is a truncated form of the predominant long versions of SEPT9 and the phenotypic effects of overexpression are consistent with a model of it acting as a dominant negative species. The association of septins with microtubules is also of interest and it may be that septins can modulate aspects of microtubule function. Some of the observations of Chacko et al (2005) point to this since (for example) polarity determination requires microtubule coordination as well as effects on the actin cytoskeleton. The possibility that SEPT9 (and possibly other septins) can alter microtubule dynamics has been proposed, and this may be of relevance to drug resistance to microtubule-acting drugs.
A final aspect of septin function that may be relevant to neoplasia is their formation of complex hetero-oligomeric structures (Versele and Thorner, 2005) and their association with the rho signalling pathway (Nagata and Inagaki, 2005). In yeast, it has been suggested that septins act as scaffolds for the recruitment and regulation of proteins involved in several processes. It may be that in man the complex array of septins and septin isoforms provides an array of cell type and context-specific spatial cues that similarly organise the spatial arrangement of other proteins, potentially in a highly regulatable manner. Perhaps the stoichiometry of particular septins and their isoforms can control such processes. Consequently, the alteration in the level of septins in cells may have profound effects. The observation that septin levels change in neoplasia and that the overexpression of one isoform (SEPT9_v4) can have profound effects fits this class of model.
Conclusion
The past 25 years have seen an explosion in our understanding of the molecular events underpinning neoplasia and more than 200 genes that are mutated in human cancers have been described (Futreal et al, 2004). However, genes whose protein products appear, at least in some tumour types, to contribute to the neoplastic phenotype continue to be identified. While it is the case that mutations are crucial to the role of many oncogenes and tumour suppressor genes in neoplasia, it is becoming increasingly apparent that the neoplastic phenotype can be a consequence of alterations in gene expression, with haploinsufficiency being an increasingly common theme (Mao et al, 2004), coupled with environmental factors, often having a multifaceted spatial (Orimo et al, 2005) and temporal interplay (Cook et al, 2005). Furthermore, the surprising revelation of how relatively few genes we have in the human genome and the extent of alternate splicing that exists, highlights the possibility that the range of genes whose products will have a role in neoplasia will continue to grow as we develop a more detailed understanding of the molecular events that regulate cells. Moreover, in some situations, the distinction between oncogenes and tumour suppressor genes may become blurred with the diverse products of one gene having different and potentially opposing functions.
The septin family of genes exemplify some of these issues and indicate how complicated the next 25 years of cancer research might be. Without question, the septin family deserves more attention and recent data suggest intriguing connections with fields as diverse as polarity control, membrane dynamics and exocytosis, the cell cycle and motility and cell shape. The consideration of septin biology and its role in neoplasia will require new perspectives and approaches to the issues of protein levels and stoichiometry, the nature and distribution of protein complexes and will ultimately require a much higher resolution analysis and with new reagents and approaches. It is also conceivable that the manipulation of septin complexes in cells may provide new insights to therapeutic options. Finally, the study of septins in neoplasia and other diseases may illuminate the broader issues of septin function in mammalian cells.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Burrows JF, Chanduloy S, McIlhatton MA, Nagar H, Yeates K, Donaghy P, Price J, Godwin AK, Johnston PG, Russell SE (2003) Altered expression of the septin gene, SEPT9, in ovarian neoplasia. J Pathol 201: 581–588
Casamayor A, Snyder M (2003) Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol Cell Biol 23: 2762–2777
Chacko A, Hyland PL, McDade SS, Hamilton P, Russell SEH, Hall PA (2005) SEPT9_v4 expression induces morphological change, increased motility and disturbed polarity. J Pathol 206: 458–467
Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL (2005) Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci USA 102: 8644–8649
Dobbelaere J, Gentry MS, Hallberg RL, Barral Y (2003) Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell 4: 345–357
Elhasid R, Sahar D, Merling A, Zivony Y, Rotem A, Ben-Arush M, Izraeli S, Bercovich D, Larisch S (2004) Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene 23: 5468–5475
Finger FP (2005) Reining in cytokinesis with a septin corral. BioEssays 27: 5–8
Finger FP, Kopish KR, White JG (2003) A role for septins in cellular and axonal migration in C. elegans. Dev Biol 261: 220–234
Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR (2004) A census of human cancer genes. Nat Rev Cancer 4: 177–183
Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S (2004) The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J 23: 1627–1635
Hall PA, Jung K, Hillan KJ, Russell SE (2005) Expression profiling the septin gene family. J Pathol 206: 269–278
Hall PA, Russell SEH (2004) The pathobiology of the septin gene family. J Pathol 204: 489–505
Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, Takahashi C, Itohara S, Nishimune Y, Noda M, Kinoshita M (2005) Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell 8: 343–352
Irazoqui JE, Lew DJ (2004) Polarity establishment in yeast. J Cell Sci 117: 2169–2171
Kalikin LM, Sims HL, Petty EM (2000) Genomic and expression analyses of alternatively spliced transcripts of the MLL septin-like fusion gene (MSF) that map to a 17q25 region of loss in breast and ovarian tumors. Genomics 63: 165–172
Kojima K, Sakai I, Hasegawa A, Niiya H, Azuma T, Matsuo Y, Fujii N, Tanimoto M, Fujita S (2004) FLJ10849, a septin family gene, fuses MLL in a novel leukemia cell line CNLBC1 derived from chronic neutrophilic leukemia in transformation with t(4;11)(q21;q23). Leukemia 18: 998–1005
Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, Book-Melamed N, Timberg R, Duckett CS, Lechleider RJ, Steller H, Orly J, Kim SJ, Roberts AB (2000) A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2: 915–921
Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, Brown K, Bryson S, Balmain A (2004) Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432: 775–779
McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Johnston PG, Russell SE (2001) Genomic organization, complex splicing pattern and expression of a human septin gene on chromosome 17q25.3. Oncogene 20: 5930–5939
Montagna C, Lyu MS, Hunter K, Lukes L, Lowther W, Reppert T, Hissong B, Weaver Z, Ried T (2003) The Septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res 63: 2179–2187
Nagata K, Inagaki M (2005) Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene 24: 65–76
Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, Inagaki M (2003) Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem 278: 18538–18543
Ono R, Nakajima H, Ozaki K, Kumagai H, Kawashima T, Taki T, Kitamura T, Hayashi Y, Nosaka T (2005) Dimerization of MLL fusion proteins and FLT3 activation synergize to induce multiple-lineage leukemogenesis. J Clin Invest 115: 919–929
Ono R, Taki T, Taketani T, Kawaguchi H, Taniwaki M, Okamura T, Kawa K, Hanada R, Kobayashi M, Hayashi Y (2002) SEPTIN6, a human homologue to mouse Septin6, is fused to MLL in infant acute myeloid leukemia with complex chromosomal abnormalities involving 11q23 and Xq24. Cancer Res 62: 333–337
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348
Osaka M, Rowley JD, Zeleznik-Le NJ (1999) MSF (MLL septin-like fusion), a fusion partner gene of MLL, in a therapy-related acute myeloid leukemia with a t(11;17)(q23;q25). Proc Natl Acad Sci USA 96: 6428–6433
Polo S, Pece S, Di Fiore PP (2004) Endocytosis and cancer. Curr Opin Cell Biol 16: 1–6
Russell SE, McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Petty EM, Kalikin LM, Church SW, McIlroy S, Harkin DP, Keilty GW, Cranston AN, Weissenbach J, Hickey I, Johnston PG (2000) Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res 60: 4729–4734
Scott M, Hyland P, McGregor G, Hillan KJ, Russell SEH, Hall PA (2005a) Multimodality expression profiling shows SEPT9 to be over-expressed in a wide range of human tumours. Oncogene 24: 4688–4700
Scott M, McCluggage WG, Hillan KJ, Hall PA, Russell SE (2005b) SEPT9 transcript expression profiling in epithelial ovarian neoplasia defines borderline tumours as a distinct category. Int J Cancer (in press).
Sorensen AB, Lund AH, Ethelberg S, Copeland NG, Jenkins NA, Pedersen FS (2000) Sint1, a common integration site in SL3-3-induced T-cell lymphomas, harbors a putative proto-oncogene with homology to the septin gene family. J Virol 74: 2161–2168
Spiliotis ET, Kinoshita M, Nelson WJ (2005) A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science 307: 1781–1785
Surka MC, Tsang CW, Trimble WS (2002) The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell 13: 3532–3545
Taki T, Ohnishi H, Shinohara K, Sako M, Bessho F, Yanagisawa M, Hayashi Y (1999) AF17q25, a putative septin family gene, fuses the MLL gene in acute myeloid leukemia with t(11;17) (q23;q25). Cancer Res 59: 4261–4265
Tanaka M, Kijima H, Itoh J, Matsuda T, Tanaka T (2002) Impaired expression of a human septin family gene Bradeion inhibits the growth and tumorigenesis of colorectal cancer in vitro and in vivo. Cancer Gene Ther 9: 483–488
Versele M, Thorner J (2005) Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol 15: 414–424
Acknowledgements
We thank the members of the Hall and Russell groups for valuable discussion. Work in these laboratories is supported by Action Cancer, the Pathological Society of Great Britain and Ireland, the Northern Ireland R&D Office, Queen's University Belfast and the Samaritan Trust. Editorially set space constraints have limited our ability to cite primary data, for which we apologise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Russell, S., Hall, P. Do septins have a role in cancer?. Br J Cancer 93, 499–503 (2005). https://doi.org/10.1038/sj.bjc.6602753
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602753
Keywords
This article is cited by
-
Septin-3 autoimmunity in patients with paraneoplastic cerebellar ataxia
Journal of Neuroinflammation (2023)
-
A multi-omics study links TNS3 and SEPT7 to long-term former smoking NSCLC survival
npj Precision Oncology (2021)
-
Cis-perturbation of cancer drivers by the HTLV-1/BLV proviruses is an early determinant of leukemogenesis
Nature Communications (2017)
-
Epigenomic profiling of prostate cancer identifies differentially methylated genes in TMPRSS2:ERG fusion-positive versus fusion-negative tumors
Clinical Epigenetics (2015)
-
An efficient protocol for the purification and labeling of entire yeast septin rods from E.coli for quantitative in vitroexperimentation
BMC Biotechnology (2013)