In the wee hours of the medieval night, a monk begins to wheeze and cry in fear. His brothers call in the healer, who brings what comfort he can. Asthma is a beast of the nighttime, the healer knows. But in this hypothetical scenario, which might have played out in thousands of darkened bedrooms and dormitories down the years, all the men can do is wait for the symptoms to pass.
Thanks to old medical compendiums, we now know that physicians have long recognized that certain medical disorders exhibit daily variations. Back in the fifth century, Roman doctor Caelius Aurelianus wrote that asthma attacks were more common after dark. In 1568 German physician Christopher Wirsung even pinpointed them as occurring between 2 a.m. and dawn. Blood pressure, heart rate, and the onset of chest pain and heart attacks were observed to have certain rhythms, too.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Of course, those astute observations are recorded alongside suggestions that have long since been relegated to folk medicine. Aurelianus, for instance, instructs readers with sore ears to coat the area with a paste made of saffron, vinegar, myrrh, quince and various other substances that may or may not have helped. Wirsung was quite adamant that foul smells were bad for the heart.
Only centuries later did scientists begin to entertain the possibility that the rhythms of the body could be harnessed for therapeutic benefit. Biologist Franz Halberg was one leader in the effort to study what he called chronobiology, the regular fluctuation of biological measurements over time in individual people and even in individual cells. But first, he and other chronobiologists had to convince their colleagues and the public that chronobiology was serious science. At the time that he gave an interview to People magazine in 1978, a system called biorhythms was all the rage—it used your date of birth to generate a trio of lines that purportedly represented an intellectual, physical and emotional status that cycled up and down. The Dallas Cowboys used biorhythms to plan game strategies. Biorhythms appeared to have predicted Clark Gable's fatal heart attack. And for a while people were even using them to time when they had sex, hoping they could influence the gender of their children.
Halberg stressed that what he studied could not be further from biorhythms, which he called “silly.” “We find cycles in every system of the body,” Halberg, who died in 2013, said to the People reporter. “Many more can be discovered, measured and eventually exploited. From the timing of a meal to the administration of an anticancer drug, working with, instead of against, the body's rhythms can tip the scale between health and disease, and even between survival and death.”
The idea initially met with considerable skepticism. It sounded grandiose. How could cancer treatment boil down to timing? The People article suggested that some of Halberg's colleagues chalked up his ideas to “paranoia.” It did not make sense to most biologists that time of day would matter. They had gotten perfectly good results in various experiments by doing them whenever it was convenient. And if some tests mysteriously did not give the same results, there could be many explanations.
Today, however, researchers know that time is a real factor in whether an experiment or a treatment succeeds or fails. They are now tracing the circuits by which the time of day writes itself on our bodies, an effort that could help doctors treat a range of diseases more effectively and safely.
Cyclic patterns of gene expression
Ueli Schibler recalls the day in 1990 when a student walked into his office and said, “You have to retract this paper. It's all fake.” At the time, Schibler, a professor at the University of Geneva, was studying what are called transcription factors. The DNA that stores all the instructions necessary for life is usually tightly bundled in a cell's nucleus. When there is a call for a particular set of instructions—that is, a gene—then proteins in the nucleus unfurl the relevant segment and transcribe it. The transcript leaves for the outer regions of the cell, where it will be read and used to build a protein. This process is called gene expression. And in it, transcription factors are key.
Transcription factors come in any number of shapes, but their uniting feature is an ability to control how and when transcription happens. They do this by attaching to the DNA itself, among other functions. They, too, are proteins made with the use of instructions encoded in genes, lending the entire picture a circular quality. A postdoctoral researcher in Schibler's laboratory had been working on isolating a transcription factor in the liver. It had all seemed to go without a hitch. He found the transcript for the protein DBP in rats, figured out the protein's sequence and identified the gene that made it. Back then, understanding how transcription factors shaped individual tissues was still in its infancy, and characterizing this powerful modulator was an exciting step forward. The researchers published a paper in the prestigious journal Cell. “He was happy, and I was happy,” Schibler says.
The postdoc went off to his new position as an assistant professor, and a student took over his project. Three months later the student dropped his bombshell: he had repeated the experiment many times, and the transcription factor was never present.
Schibler found it hard to believe his postdoc guilty of fraud, but what other options were there? He immediately tried the experiment himself. This time, to Schibler's mystification, DBP appeared. The researchers looked at every variable, and eventually they alighted on something odd. The postdoc had performed the experiments with the rats' livers in the early afternoon. Schibler had also done his experiment in the afternoon. But the student was a farmer's son who came in around 7 a.m. and did the work in the morning.
It turned out that when the student looked for the transcription factor, it was simply not present at detectable levels. At the time, researchers generally assumed that genes made their protein products more or less consistently at all hours of the day. But the gene that made DBP was on a 24-hour cycle that repeated every day: nearly none of the protein was made in the morning, but by the afternoon, levels spiked 300-fold. Schibler and his collaborators described this surprising pattern in a second Cell paper published later that same year.
In the decades since, researchers from all over the world have found that genes whose expression patterns have daily highs and lows are not aberrations. In the late 1990s researchers studying cyanobacteria, which are photosynthetic, found that more than 80 percent of the microbes' genes produce their proteins according to a circadian, or daily, rhythm. That discovery made sense because these organisms are tied so strongly to the sun, but it soon became clear that many genes in flies and mice were oscillating as well. A 2014 paper by John Hogenesch, now at Cincinnati Children's Hospital Medical Center, and his colleagues took a closer look at the phenomenon, tracing the expression of nearly 20,000 genes across 12 different tissues in mice. The team recorded gene expression levels every two hours and realized that there were rush hours when large numbers of genes became active, just before dawn and just before dusk. Furthermore, when the researchers looked at how many genes in total had a cyclic pattern, the proportion came to a whopping 43 percent of the genome.
That study has since been cited more than 450 times, according to Google Scholar, as the trickle of papers involving circadian gene expression has become a flood. The latest estimate of genes that run by the clock in mammals, from work on nonhuman primates by Satchidananda Panda of the Salk Institute for Biological Studies in La Jolla, Calif., and his collaborators that was published in February in Science, is even higher: 82 percent of genes—a difference that Panda attributes in part to having sampled many more tissues in his study. “That completely changes things,” Panda says. “That means there is a time aspect to the genome.”
Imagine the body as a Rube Goldberg machine, with thousands of tiny devices whose cogs, baskets and springs must align correctly in a moment for life to proceed. And it turns out that not all the springs or baskets are present at any given moment. If you send a marble down a chute, the route it takes in the morning may be different from the route it takes in the evening.
The conductor of all this timed expression is the circadian clock, which is not a single object or place in the brain, as the name might lead you to believe, but a squad of about a dozen proteins. At the same time that some researchers were uncovering cycles in gene expression, others were revealing the clock side of the mystery. We now know that the clock proteins' own levels rise and fall over the course of the day, thanks to directions from a light-sensitive region in the brain, the circadian pacemaker. The clock proteins help to drive the expression of all the other genes that cycle daily, pushing the buttons and pulling the rods that bring some proteins into play and switch others off, regulating everything from cell division to metabolism. They are present in nearly every cell in the body.
The significance of this work is growing: The 2017 Nobel Prize in Physiology or Medicine went to three circadian clock researchers who discovered a central clock protein that builds up in cells during the night, breaks down during the day and acts as a kind of crankshaft for the whole machine. The findings of circadian clock researchers imply that on the level of the organism, there is a good time and a bad time to do anything, especially when it comes to medical intervention. But when, exactly?
Poison or cure?
Acetaminophen, marketed under several brand names, including Tylenol, is a danger in disguise. It is a painkiller for the most innocent of uses—headache, muscle soreness—but when too much is taken, the liver can be damaged. In a handful of days, if the overdose is not treated, the patient may die. Acetaminophen overdose is behind more than 78,000 emergency room visits a year in the U.S.
Could it be that some of acetaminophen's peculiar lethality has to do with when people take it? Chronopharmacologist Robert Dallmann of the University of Warwick in England and his colleagues have found intriguing evidence in mouse studies that the answer is yes. When you give mice a dangerously large dose in the morning, absolutely nothing untoward happens. “You give it in the evening,” Dallmann says, “and the liver is basically kaput.”
Click or tap to enlarge
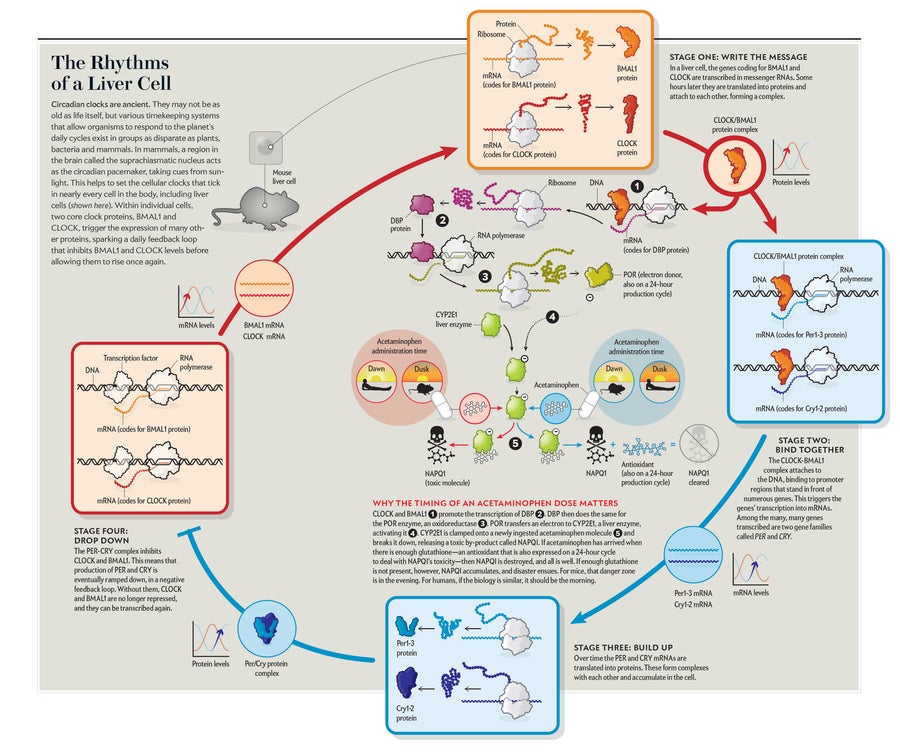
Credit: Tami Tolpa
This is the way it works: As the two proteins at the heart of the clock, called CLOCK and BMAL1, go through their daily cycle in the liver, they flip a switch and cause the transcription of DBP. DBP, in turn, causes the expression of the POR enzyme. POR acts on yet another enzyme, one that pharmacologists are familiar with: CYP2E1, which is one of the liver enzymes that take apart drugs, alcohol and substances in food. Such liver enzymes ramp up their levels in the morning in humans and in the evening in mice, which are nocturnal. Schibler suggests the enzymes are being prepared for the times when the organism is most likely to eat.
POR hands off an electron to CYP2E1. If the person—or mouse—has recently swallowed acetaminophen, CYP2E1 clamps onto the drug molecule. A series of swift, small changes (moving an oxygen molecule in, executing a sleight of hand with protons and another electron) results in the release of water—and a piece of what used to be acetaminophen is now an extremely dangerous poison.
Most of the time, the poison does not stick around. As the cytochrome releases the poison, it is caught by another enzyme and broken down into something harmless by an antioxidant. But that antioxidant also happens to be produced on a schedule set by the circadian clock. If acetaminophen arrives when the cytochrome is present and there is not enough antioxidant to handle it, the poison accumulates, and disaster ensues. For mice, that danger zone is the evening. For humans, if the biology holds the same, it would be in the morning.
Intriguingly, destroying the clock eliminates the deadly differences between morning and evening. “We can show that if we turn off the clock in the liver, this rhythm is gone,” Dallmann says. In these clockless cells, genes are expressed in a more disorganized manner. Imagine, then, a Rube Goldberg machine that has roughly all its parts in play at once, in a kind of genetic cacophony that probably cannot be maintained for long if the animal is to stay healthy. But the mouse experiment does prove that the circadian clock is key to the effects of the drug.
Circadian influence on common drugs
If such an enormous portion of the genome makes proteins only at certain times of day and if drugs interact with those substances, then the time of dosing probably matters with more medications than acetaminophen. In fact, 56 of the 100 most commonly used drugs in the U.S. target rhythmically expressed proteins, Hogenesch and his colleagues reported in their 2014 paper. About half of those drugs have a half-life in the body of less than six hours, suggesting that timing the dose could make a difference in their effectiveness. Aspirin prescribed to ward off heart attacks, for instance, has only a short half-life in the body. But the enzyme it targets showed a daily cycle in heart, lung and kidney tissues in that study. Perhaps that pattern explains the results of a 2005 trial in patients with hypertension that showed that taking aspirin before bedtime lowered blood pressure, whereas taking the drug in the morning slightly elevated it. A smaller, randomized human study in 2014 showed that aspirin before bedtime caused a decrease in a kind of blood cell activity that leads to blood clots. Aspirin in the morning did not.
In addition to exploring the circadian influence on common medications, many researchers who are now interested in the idea of so-called chronomedicine—which is timing treatment for maximum safety and efficacy—have focused on some particularly dangerous drugs: those used in treating cancer. Chemotherapies can cause potent side effects and permanent harm in some patients. The biochemical processes that create these side effects have something in common with what happens to acetaminophen. Like acetaminophen, some chemotherapies can interact with liver enzymes that are under circadian control, and their efficacy sometimes seems to depend on the time of day when they are administered. Decades ago Halberg and his colleagues found that whether mice with cancer lived or died came down to the hour when they received their medicine, relates Germaine Cornelissen of the University of Minnesota, who came to work with him shortly after.
Francis Levi, a chronobiologist and medical oncologist at the University of Warwick, and his collaborators have been performing rigorous tests of the idea for more than 20 years. In one landmark study, Levi and his colleagues looked at what happened in 93 patients with colorectal cancer when they got their medicine at a particular time. In human cells, the enzyme dihydropyrimidine dehydrogenase is responsible for safely breaking down the chemotherapy drug fluorouracil. The enzyme's levels spike by nearly 40 percent around midnight. If patients could get their drugs then, the researchers reasoned, they might see fewer painful, dangerous side effects. Indeed, the scientists found a fivefold reduction of inflammation in the mucosal membranes and a threefold reduction in hospitalizations for side effects.
In another trial, the team found men with colorectal cancer survived longer with timed treatment, but women did not. “[The finding] does not mean that women do not benefit from chronotherapy,” Levi says, “just that the timing in women has to be different from timing in men.” With this particular drug, it turned out that at least in mice, the clock proteins of females cycle differently enough from those of males that the optimum treatment time was hours off. Levi and his collaborators have, with a biomedical device company, developed pumps that inject a dose at a predetermined time, even if it is when the patient or the doctors are asleep.
But if these data have been published for decades, why is chronomedicine not more widely practiced? For one thing, not all trials have seen an effect. It is hard to tell whether that is because experimenters are not looking at enough times of the day or some other variable or because there is simply nothing to be gained by timing a dose. For much of the time in which Levi and other pioneers were working on this issue, the mechanistic details of how the circadian clock functioned and exactly how it influenced what happens after you take a drug were still uncertain.
New insights may raise the field's profile. In March researchers based in China and the U.S. outlined just how the circadian clock functions in 32 different kinds of cancer. And in another recent paper, Hogenesch and his colleagues found that giving a chemo drug at the right time, when enzymes can whisk away dangerous by-products, halves its toxicity in mice.
Meanwhile mounting evidence supports the use of timed treatment for other diseases, including inflammatory and autoimmune disorders such as rheumatoid arthritis, whose sufferers have long complained of swollen, sore joints in the morning. We now know that the circadian clock is driving inflammation in the joints at that time, says Julie Gibbs of the University of Manchester in England, who studies the phenomenon. A timed-release formulation of a drug engineered to be present before waking has shown striking success in a clinical trial by another group, she says. Even the blood-brain barrier may be more permeable at certain times: Amita Sehgal and her lab at University of Pennsylvania just reported in experiments with fruit flies that seizure medications are most effective at night, because molecular pumps that remove drugs from the brain have cyclical expression.
Thanks to the snowballing number of papers on the circadian clock, this research is no longer driven by trial and error. “Efforts over the past 40 years were largely serendipitous,” Hogenesch says. “We now have principled, mechanism-based strategies.” In other words, researchers can plan for the fact that the body runs on a timetable.
Potential for personalized medicine
Pharmaceutical companies and clinicians have not responded with the same level of fervor as scientists—at least not yet. Human tests of the molecular mechanisms uncovered in animals have been slow to emerge. And chronobiology is not generally studied in medical school, which means that the people who might reasonably use this information do not know much about it. From the drug companies' perspective, time of day is an expensive thing to control for. Imagine doing double the testing, just to see if morning and evening have different effects—not to mention all the times in between.
Further complicating matters, some evidence indicates that optimum dosing time can vary significantly among individuals. Although we are all running on roughly the same schedule, some people are a little slower or faster. Cornelissen and her colleagues in Japan have found that in people with high blood pressure, personalized monitoring of circadian rhythms can determine different optimum treatment times for each person. Levi's cancer studies, too, have found that studying patients' own personal daily cycle is important. This level of detail can seem bewildering.
Drug companies have already tripped over the circadian clock. Back in the 1980s, Levi says, a drug company tried to reduce the side effects of an anti-inflammatory that can cause stomach problems by introducing a delayed-release formulation. “It was a real disaster,” Levi recalls. Despite all of the company's work, the rate of severe side effects did not go down. At its request, Levi ran a trial with around 500 patients and found that the drug was actually most toxic in the morning, when the new pill was designed to be taken, probably as a result of liver enzyme cycling. Checking for such effects before a drug is put on the market could be a way to avoid such snafus, as well as potentially improving efficacy.
Indeed, individual variation may not be just an inconvenience; it may also be an opportunity for pharmaceutical companies. Personalized medicine—the idea that treatment can be engineered specifically to the patient—is on the rise, and the goal of incorporating the clock dovetails with it. If one can eliminate side effects in a particular group of people by assigning them a time or giving them a formulation that only kicks in later, then that is a net benefit for a drugmaker. To that end, it is getting much easier to tell where someone is in their daily cycle with simple, noninvasive tests. Hogenesch, for instance, is currently investigating an assay using cells picked up on a cotton swab swiped across a patient's skin.
Will we someday go into the doctor's office knowing the details of our own clock the way we know our blood type? Will we receive a customized time card for when to take our meds? Answers vary, but “I really think so,” Schibler says.
Because if we could go back in time to the asthmatic monk in the monastery, modern pulmonologists could explain that one reason asthma attacks tend to occur in the wee hours may be because that is when certain hormones on a circadian cycle spike. They shrink the passageways in the lungs, triggering a crisis in some people with asthma, and a drug called theophylline can reduce that effect. Today it is taken before bed in a capsule that dissolves over time so that it will be there in the blood when it is needed some hours later. More than 1,500 years after Caelius Aurelianus wrote that asthma came at night, we have some answers about time and the body—as well as new mysteries to solve.”

