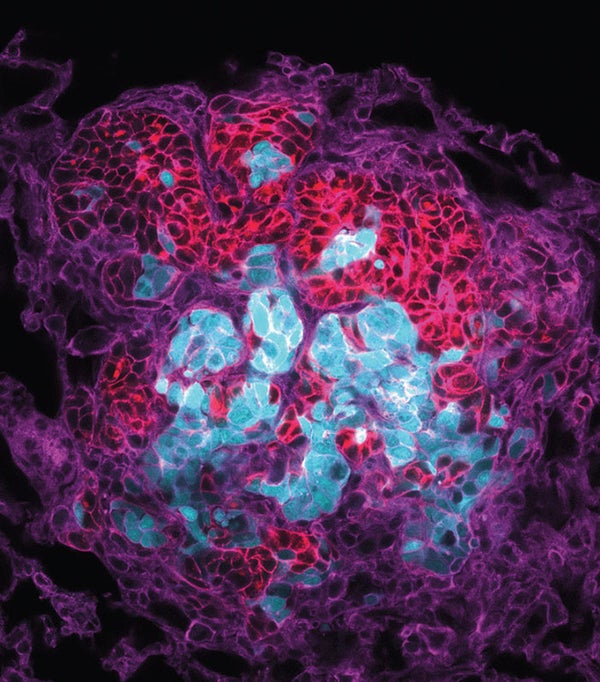
Metastasis is behind the vast majority of cancer deaths: when cancer cells break away from a tumor and lodge in new places, the disease becomes harder to treat. A new study shows that, contrary to expectations, most metastatic tumors are seeded not by single cells from the primary tumor but by clusters of diverse cancer cells that leave in a group and travel through the bloodstream together. The cells in these circulating clusters communicate with one another and produce specific proteins that could be used as drug targets or biomarkers for risk of metastasis.
To determine how metastases form, cancer cell biologist Andrew Ewald and his team at Johns Hopkins University created tumors in mice by injecting a mixture of multicolored cancer cells into the rodents' mammary glands. If tumors originated elsewhere from single cells, then they would show up under the microscope as one uniform color. If instead tumors were seeded by clusters of cells, then they would grow into rainbow-colored balls. The team found that about 95 percent of the cancers that formed were in fact multicolored and therefore derived from multiple cells (lung metastasis, above).
In a second experiment, the researchers examined hundreds of cancerous cells grown together in a petri dish but placed so that they were not touching. Almost all of them died. In contrast, cells in another dish that were aggregated into clusters subsequently formed more colonies—even though there were fewer “seeds” to begin with. “Controlling for cell number, there is more than a 100-fold increase in efficiency of metastasis formation in the aggregated cells,” Ewald says. The findings were published in February in the Proceedings of the National Academy of Sciences USA.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
It is not yet entirely clear why the aggregated cells survive and metastasize more effectively, but it is likely that cooperation among tumor cells within clusters—for example, exchanging signaling molecules—protects against cell death in the bloodstream or at distant sites, explains Joan Brugge, a cancer cell biologist at Harvard Medical School who was not involved in the study.
As for potential benefits to patients, Ewald's team also found that the traveling clusters share molecular features and nearly all make the protein keratin 14. “We could potentially use this [insight] to develop targeted ways to attack all the metastatic cells,” Ewald says. The idea would be to wipe out those cells wherever they are in the body, whether or not they are proliferating—a different approach from most standard therapies, which focus on attacking rapidly proliferating cells but not the circulating, invasive ones that initiate secondary cancers.