Abstract
Background
Many studies have characterized racial differences in cancer outcomes, demonstrating that black and Hispanic patients have lower cancer-specific survival compared to white patients. However, to our knowledge, a gap in the literature exists regarding racial, socioeconomic, age, and sex-related differences in survival improvement in cancer.
Methods
We perform a population-based cohort study of 1,875,281 patients with eight common cancer sites from the Surveillance, Epidemiology, and End Results (SEER) database.
Results
The longitudinal data demonstrates that while overall cancer-free survival has improved from 2004 to 2018, certain groups have seen lower rates of improvement. Black patients have the lowest cancer-specific survival (CSS) in breast, prostate, ovarian, colon, liver, lung, and pancreatic cancers. However, from 2009 to 2018, black patients have seen the greatest survival improvement in breast, ovarian, colorectal, liver, lung, and pancreatic cancer, though CSS for black patients still lags behind other groups. Strikingly, however, in breast and prostate cancer, black patient CSS from 2014 to 2018 remains lower than white patient CSS from 2004 to 2008 after controlling for income, age, and stage.
Conclusions
While the racial disparity gap is closing in some forms of cancer, future research should focus on identifying factors causing disparate outcomes to help reduce cancer-related disparities.
Similar content being viewed by others
Introduction
Cancer is the second leading cause of human deaths in the US and many other countries, causing around 10 million deaths worldwide per year [1, 2] Different cancer sites are typically associated with different survival, with favorable survival time in breast and prostate cancer and unfavorable survival time in lung and pancreatic cancer [2]. During the past two decades, cancer treatment options have greatly advanced, resulting in significantly improved survival in recent years [3] Notably, however, survival improvements vary across different cancer sites.
In the same cancer type, the survival of different patient subsets can also vary dramatically depending on race, age, sex, and socioeconomic status, a phenomenon known as cancer disparity. Studies have reported worse survival for black patients compared to white patients in breast [4] and prostate [5] cancer and better survival for females compared to males in lung cancer [6, 7]. Additionally, young age at diagnosis and high socioeconomic status are commonly associated with favorable prognosis in nearly all cancer sites [8, 9].
Although disparities in survival have been extensively investigated across cancer sites, disparities in survival improvements remain poorly studied. Investigating such disparities is important as it may help us understand the underlying causes and forecast the future trend of survival disparities. Based on our findings, strategies such as optimizing treatment options and reallocating healthcare resources can be used to reduce survival disparity problems. A previous study revealed disparities in survival improvements for patients diagnosed between 1990 and 2009, with significant survival disparities between age groups and narrowed survival disparities between racial groups, except for ovarian cancer [10]. In the past decade, the survival of almost all cancer sites has improved substantially [3, 11, 12] thanks to the availability of new treatment options, especially the wide application of immunotherapies [13, 14]. Our previous work has also demonstrated survival improvement in prostate cancer, but the results have not yet been expanded and compared across additional cancer sites [15]. As such, it is critical to revisit the racial disparity issue in the context of survival improvement across different cancer sites. In this study, we used population-based cancer registry data collected by the Surveillance, Epidemiology, and End Results (SEER) database to evaluate survival improvements in different patient subsets according to race, age, socioeconomic status and sex in eight cancer sites from 2004–2018.
Methods
SEER database
We analyzed SEER 18 registries Incidence-Based Mortality data for cancer patients diagnosed during 2004–2018. Eight cancers of the most common cancer sites were selected for this study (Supplementary Table 1). To ensure high data quality, we applied the following criteria to select patients: (1) the “Type of Reporting Source” is “Hospital inpatient/outpatient or clinic”, (2) the patient is diagnosed with only one primary cancer as indicated by “One primary only” in the variable “Sequence number” provided by SEER, (3) age of diagnosis is between 40 and 85.
The overall and cancer-specific survival (CSS) information was determined based on the variables “Vital status recode (study cutoff used)”, “SEER cause-specific death classification” and “Survival months”. The race information was determined by the variable “Race and origin recode (NHW, NHB, NHAIAN, NHAPI, Hispanic)”, from which “Non-Hispanic White”, “Non-Hispanic Black”, “Non-Hispanic Asian or Pacific Islander”, and “Hispanic (All Races)” were categorized as White, Black, Asian, and “Hispanic”. Of note, Hispanic is an ethnic category and not considered a racial category. Except for breast cancer, cancer stages were determined based on “Derived AJCC Stage Group, 6th ed (2004–2015)”, “Derived SEER Cmb Stg Grp (2016–2017)”, and “Derived EOD 2018 Stage Group (2018+)” for patients diagnosed during 2004–2015, 2016–2017, and 2018, respectively. For breast cancer, cancer stages were determined based on “Breast - Adjusted AJCC 6th Stage (1988–2015)”, “Derived SEER Cmb Stg Grp (2016–2017)”, and “Derived EOD 2018 Stage Group (2018+)” for patients diagnosed during 2004–2015, 2016–2017, and 2018. The age, sex and annual income information were determined based on “Age recode with single ages and 85+”, “Sex” and “Median household income inflation adj to 2019”, respectively. Of note, the prostate cancer cohort in our previous study [15] was 534,076 and 506,717 in the present study.
Statistical analysis
We performed survival analysis focusing on CSS with R package “survival”. Multivariable Cox regression was used to estimate survival disparities in different patient subgroups according to race, age, income and sex. Specifically, we stratified patients into three age groups: 40–55 (Younger), 56–70 (Middle), and 71–85 (Older) groups; and three income groups: “< $60,000” (Low), “$60,000–$74,999” (Intermediate), and “>$75,000” (High). Income groups were selected to straddle the median US income of ~$70,000. To investigate racial, age, socioeconomic and sexual disparities, we used white race, younger age, low income and female sexes as reference groups, respectively.
To investigate survival improvement disparities, we grouped patients into three 5-year bins according to their year of diagnosis: 2004–2008, 2009–2013, 2014–2018; and used 2004–2008 as the reference group. We used multivariable Cox regression to calculate hazard ratios (HRs) and 95% CIs for each 5-year survival increment in different patient subgroups according to race, age, income and sex. P values less than 0.05 were considered as significant.
Results
Cancer-specific survival patterns by race, age, income, and sex
We investigated CSS after 1, 3, and 5 years for each cancer type, stratified by race, age, income, and sex (Supplementary Table 2). White patients have the highest CSS in breast, prostate, and rectal cancers, while Asian patients have the highest CSS in ovarian, liver, lung, and pancreatic cancers. Black and Hispanic patients have the lowest CSS in breast and prostate cancers, and black patients also demonstrate the lowest CSS in ovarian, colon, liver, lung, and pancreatic cancers.
As expected, the oldest age group, age 71–85 at diagnosis, has the worst CSS in all cancer sites, while the youngest group, age 40–55 at diagnosis, has the highest CSS in all cancer sites. High-income patients have the highest CSS in ovarian, liver, lung, and pancreatic cancers but the lowest CSS in breast and prostate cancers. Finally, female patients overall have higher CSS in all cancers, except for rectal cancer, when compared to male patients.
Racial disparities in cancer-specific survival for a wide array of cancers
We performed stratified cross-sectional analyses to determine the effects of race, age at diagnosis, socioeconomic status, sex, and cancer stage for each cancer type (Fig. 1). Our analyses indicated that for all cancer sites, except lung cancer, black patients demonstrate a significantly lower CSS when compared to white patients, with the highest disparity observed in breast cancer. Asian patients have a significantly longer CSS in all cancer sites. For lung cancer in particular, Asian patients have the highest survival advantage (adjusted HR = 0.73, CI = [0.72, 0.74]). Hispanic patients have significantly higher CSS in lung cancer (adjusted HR = 0.92, CI = [0.90–0.93]), but differences in CSS are not significant in the other cancer sites. Additionally, a cross-sectional age disparity was observed in all cancer sites. In breast, ovarian, prostate, colon, rectal, lung, and pancreatic cancer, the 56–70 and 71–85 age groups show an increased risk of cancer-specific death when compared to the younger group (40–55).
Significant socioeconomic disparities with consistent patterns were observed in all cancer sites. With the increase of adjusted annual household income, patients show a lower risk of CSS for all cancer sites. Finally, in all cancer sites affecting both men and women, male patients have a significantly higher HR when compared to female patients. Furthermore, as expected, in all cancer sites, diagnosis with more advanced stages is associated with lower CSS. In most cancers, Stage IV shows a higher HR than the other three stages.
Overall cancer-free survival has improved from 2004 to 2018, though disparities remain
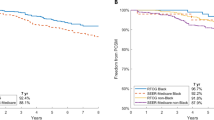
Next, we examined the improvement in survival for by race for each 4-year period between 2004 and 2018. Our results indicate that all races show an improvement in survival for most cancer sites between 2009–2013 and 2014–2018 when compared to 2004–2008 (Fig. 2). White patients experienced a significant improvement in CSS in all cancer sites in each time period. A similar improvement was seen in Hispanic patients, with a significant improvement in survival in all cancers and time periods, except for prostate cancer survival. Asian patients also demonstrated significant improvements in survival for most cancer sites, except for colon cancer. Black patients experienced the greatest improvement in CSS comparing 2014–2018 with 2004–2008 in breast, ovarian, colon, rectal, liver, lung, and pancreatic cancer. However, in breast, ovarian, and prostate cancer, black patients from 2014 to 2018 still demonstrate lower survival than white patients from 2004 to 2008, suggesting a persistent disparity in these three cancer sites.
Younger patients demonstrate improved survival across many cancer sites
We next examined changes in CSS due to age at diagnosis between 2004 and 2018. Notably, significant improvements in survival were achieved for all age groups in all cancer sites (Fig. 3). In colon, lung, and pancreatic cancers specifically, the youngest group achieved the greatest improvement in survival while in ovarian and liver cancers, the older groups achieved the greatest survival improvement. It is notable that for colon and rectal cancer, the middle age group 56–70 had the lowest improvement when compared to the younger and older age groups.
Higher income patients demonstrate improved survival across many cancer sites
In addition to age and race, we investigated the changes in socioeconomic disparity from 2004 to 2018. Our results indicated that in most cancer sites, except for breast and liver cancer, patients in the high-income group (>75 k) exhibited the greatest survival improvement, especially from 2014 to 2018 (Fig. 4). Of note, prostate cancer patients with a high income achieved a CSS increase by 27% (adjusted HR = 0.73, CI = [0.68–0.78]), compared with 17 and 13% achieved by the low- (adjusted HR = 0.83, CI = [0.78–0.89]) and intermediate-income (adjusted HR = 0.87, CI = [0.82 = 0.93]) patient groups. In breast and liver cancers, all income groups achieved a similar survival improvement across 2004–2018.
Sex-based differences in cancer survival improvement from 2004–2018
We also examined changes CSS by sex from 2004 to 2018. In colon, rectal, liver, and lung cancers, female patients achieved greater improvements in CSS when compared to male patients, while in pancreatic cancer, male patients achieved greater survival improvements than female patients (Fig. 5).
Disparity in prognosis improvement may be partially explained by earlier diagnosis
Finally, we compared the percentage of patients diagnosed at each cancer stage from 2009 to 2013 versus 2004–2008 and from 2014 to 2018 versus 2004–2008 (Table 1). In the latter comparison, black patients demonstrated an increase in stage I diagnosis across all eight cancer sites and a decrease in stage IV diagnosis in breast, rectal, liver, and pancreatic cancers. Across most racial groups and except for prostate, colon, and ovarian cancer, an increase in patients diagnosed at stage I and a decrease or stability in patients diagnosed at stage IV was seen. The trend is particularly clear in liver and pancreatic cancers, where all four groups experienced an increase in stage I diagnoses and a decrease in stage IV diagnoses comparing 2014–2018 to 2004–2008 data. In prostate cancer, a decrease was seen in stage II diagnoses, but an increase was seen in stage I, III, and IV diagnoses.
Discussion
Using SEER data from 2004 to 2018, we investigated changes in racial, socioeconomic, age, and sex disparities in cancer survival for eight different cancer sites. Healthcare access and equity is an important issue in cancer research, and our present study demonstrated persistent racial differences in CSS for each of our eight studied cancer sites. For example, white patients have the highest CSS in breast, prostate, and rectal cancers while Asian patients have the highest CSS in ovarian, liver, lung, and pancreatic cancers. We found that black patients have significantly lower CSS in most cancer sites when compared to white patients after adjusting for age, cancer stage, and income, while Asian patients have significantly longer CSS in most cancer sites when compared to white patients, which is consistent with previous studies [16, 17]. One driver of these disparities may be due to differences in medical treatment and care for specific races despite efforts to equitize healthcare access. One study found that white patients were more likely to receive aggressive treatment for colorectal cancer [18]. Other studies have shown that African Americans with stage I/II non-small cell lung cancer are less likely to receive recommended treatment of surgery compared to white patients, even after controlling for income level and insurance status [19, 20]. Notably, while patients of all races experienced a significant improvement in CSS in 2009–2013 and 2014–2018 when compared to 2004–2008, black patients with breast, ovarian, and prostate cancer from 2014 to 2018 demonstrate lower survival than white patients from 2004 to 2008, despite experiencing improvements over time. Our study demonstrates that a clear and persistent racial difference in CSS is still seen, highlighting the need to improve care for specific patient populations.
Most cancers arise in patients over the age of 60, and due to advances in healthcare, the worldwide population is aging, with 20% of the world’s population estimated to be over the age of 60 by 2050 [21]. In our study, age disparities were noted for all cancer sites, with patients being diagnosed with cancer at an older age having a significantly worse CSS than patients diagnosed between the ages of 40 and 55. These results are consistent with a 2010 study of the SEER database, which found a lower CSS in all analyzed cancers in older patients, except in leukemia and Hodgkin lymphoma [22]. Older patients have higher rates of comorbidities, such as diabetes, high cholesterol, and hypertension, in addition to reduced immune function, making them susceptible to increased morbidity and mortality with concurrent cancer diagnoses. Additionally, molecular studies have demonstrated that the ageing microenvironment may play a role in the differential outcomes of young versus old patients in various forms of cancer [21]. Future basic and translational clinical research studies may benefit from more clearly outlining the differences between younger and older cancer patients to help improve therapy and outcomes in older patients, a group that our study has demonstrated suffers from lower CSS.
In addition to race and age, socioeconomic status, sex, and stage are additional important epidemiological and clinical factors that help us understand differences in CSS. In the present study, we found that patients with higher adjusted annual household incomes have higher CSS than patients with lower income levels, male patients have poorer CSS than female patients in most cancer types, and patients diagnosed at Stage IV have lower CSS than those diagnosed at an earlier stage. One study found that cancer death rates in men and women were 13 and 3% higher, respectively, in poorer counties compared to wealthy counties [23]. This trend may be partially explained by the fact that wealthier patients are significantly more likely to receive preventative cancer screening [24], and they may also have access to additional treatment modalities and care options [25]. This disparity highlights a need to improve access and affordability, increase preventative cancer screening in vulnerable populations, and reduce barriers to care [25]. Our results also suggest that socioeconomic disparities in CSS have grown wider between 2004 and 2018. For most cancer sites, except for breast and liver cancers, the highest income group achieved greater improvements in survival than the lower and middle income groups.
Finally, our present study also sought to characterize differences in survival improvement by race. From 2009 to 2018, for example, black patients have also seen significant survival improvements in each of the studied cancers. Part of this may be explained by increases in stage I diagnoses for black patients across all eight studied cancer sites and decreases in stage IV diagnoses for four cancer sites. While there still exist differences in overall survival, it appears that early diagnosis can help improve cancer mortality, especially in populations with lower overall survival. The present analysis also has several limitations, including the presence of unrecorded variables, variations in data coding and reporting, patient migration between SEER registry areas, missing data, and the potential for early censoring to indicate worse survival [26]. Another significant limitation is that the hospital-based data from SEER does not capture patient data from private clinics, outpatient radiation centers, nursing homes, or other outpatient physician offices, which are common treatment sites for certain cancers, such as breast and prostate cancer. Furthermore, certain patient populations may experience a higher rate of loss to follow-up and inflated survival. Additionally, the relationship between molecular receptors and race/ethnicity was not included in the present study due to data availability of molecularly defining receptors across all cancer sites, but analysis should be conducted in the future with datasets containing the necessary molecular data. Finally, income was calculated as an area-based measure, which is imperfect and can introduce inaccuracies, especially in large counties that may have a wide range of individual incomes.
In conclusion, analysis of the SEER data demonstrates persistent significant racial and socioeconomic disparities in cancer survival for all cancer sites. While racial disparities may have been reduced due to greater differential improvement of cancer survival in black patients when compared to patients of other races, black patients with breast, ovarian, and prostate cancer may remain at an increased risk. Additionally, socioeconomic disparities have widened as patients with a high adjusted household income achieved greater survival improvements than patients of low and middle incomes. Additionally, our present study provides an atlas to help view macro-level trends in cancer survival. The slower improvement in survival seen in certain cancers, such as prostate cancer, may be due to earlier advances that improved patient survival, while recent advances or trends, such as decreased smoking patterns in the US, have led to improved recent survival in other cancers (e.g., lung cancer). Taken together, our results are encouraging as improved survival was seen broadly across most cancer sites, and future studies may aim to focus on highly morbid cancers that have seen less improvement than other cancer sites over the years. Importantly, our study also indicates that more resources and interventions should be implemented to improve cancer treatment for specific patient groups with lower CSS.
Data availability
Data is publicly available and accessible through the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. https://doi.org/10.3322/caac.21731.
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–48. https://doi.org/10.3322/caac.21412.
Chowdhury-Paulino IM, Ericsson C, Vince R, Spratt DE, George DJ, Mucci LA. Racial disparities in prostate cancer among black men: epidemiology and outcomes. Prostate Cancer Prostatic Dis. 2022;25:397–402. https://doi.org/10.1038/s41391-021-00451-z.
Oberaigner W, Siebert U. Do women with cancer have better survival as compared to men after adjusting for staging distribution? Eur J Public Health. 2011;21:387–91. https://doi.org/10.1093/eurpub/ckq099.
Davuluri S, Bajpai AK, Thirumurugan K, Acharya KK. The molecular basis of gender disparities in smoking lung cancer patients. Life Sci. 2021;267:118927. https://doi.org/10.1016/j.lfs.2020.118927.
Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. https://doi.org/10.1155/2017/2819372.
Lortet-Tieulent J, Georges D, Bray F, Vaccarella S. Profiling global cancer incidence and mortality by socioeconomic development. Int J Cancer. 2020;147:3029–36. https://doi.org/10.1002/ijc.33114.
Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the national cancer institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. https://doi.org/10.1001/jamaoncol.2014.161.
Santucci C, Carioli G, Bertuccio P, Malvezzi M, Pastorino U, Boffetta P, et al. Progress in cancer mortality, incidence, and survival: a global overview. Eur J Cancer Prev. 2020;29:367–81. https://doi.org/10.1097/CEJ.0000000000000594.
Hulvat MC. Cancer incidence and trends. Surg Clin North Am. 2020;100:469–81. https://doi.org/10.1016/j.suc.2020.01.002.
Suda K. Recent Advances in Cancer Immunotherapy. Biomolecules. 2021;11. https://doi.org/10.3390/biom11020335.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96. https://doi.org/10.1038/s41573-018-0006-z.
Zhang B, Li J, Tang M, Cheng C. Reduced racial disparity as a result of survival improvement in prostate cancer. Cancers. 2023;15:3977. https://doi.org/10.3390/cancers15153977.
Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–73. https://doi.org/10.1001/jama.2014.17322.
Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA Netw Open. 2020;3:e202950. https://doi.org/10.1001/jamanetworkopen.2020.2950.
Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. https://doi.org/10.1093/jnci/94.5.334.
Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. https://doi.org/10.1056/NEJM199910143411606.
Singh G, Miller B, Hankey B, Edwards B. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975 - 1999. Surveillance Research Program, Division of Cancer Control and Population Sciences.
Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20:89–106. https://doi.org/10.1038/s41568-019-0222-9.
Bassily MN, Wilson R, Pompei F, Burmistrov D. Cancer survival as a function of age at diagnosis: a study of the surveillance, epidemiology and end results database. Cancer Epidemiol. 2010;34:667–81. https://doi.org/10.1016/j.canep.2010.04.013.
Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. https://doi.org/10.3322/canjclin.54.2.78.
Rajaguru V, Kim TH, Shin J, Lee SG. Income disparities in cancer screening: a cross-sectional study of the Korean National Health and Nutrition Examination Survey, 2013–2019. Front Public Health. 2022;10:820643 https://doi.org/10.3389/fpubh.2022.820643.
Pramesh CS, Badwe RA, Bhoo-Pathy N, Booth CM, Chinnaswamy G, Dare AJ, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med. 2022;28:649–57. https://doi.org/10.1038/s41591-022-01738-x.
Jairam V, Park HS. Strengths and limitations of large databases in lung cancer radiation oncology research. Transl Lung Cancer Res. 2019;8:S172–S183. https://doi.org/10.21037/tlcr.2019.05.06.
Acknowledgements
This study is supported by the Cancer Prevention Research Institute of Texas (CPRIT) (RR180061 to CC and RR170048 to CA) and the National Cancer Institute of the National Institute of Health (1R01CA269764 to CC). CC and CA are CPRIT Scholars in Cancer Research.
Funding
This study is supported by the Cancer Prevention Research Institute of Texas (CPRIT) (RR180061 to CC) and the National Cancer Institute of the National Institutes of Health (1R01CA269764 to CC). CC is a CPRIT Scholar in Cancer Research.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conceptualization, investigation, data curation, and formal analysis. VS and BZ contributed to writing the original draft, and VS, CA, and CC contributed to writing, reviewing, and editing the final draft. CC supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaw, V., Zhang, B., Tang, M. et al. Racial and socioeconomic disparities in survival improvement of eight cancers. BJC Rep 2, 21 (2024). https://doi.org/10.1038/s44276-024-00044-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44276-024-00044-y