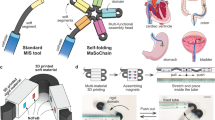
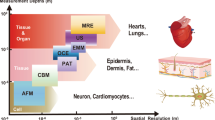
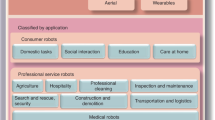
Abstract
Small-scale wireless soft robotics can be designed as implantable, interventional or wearable devices for various biomedical applications. Their flexibility, dexterity, adaptability and safe interactions with biological environments make them promising candidates for enabling precise and remote healthcare and disease diagnosis. However, the clinical translation of wireless soft robotic medical devices remains challenging. In this Review, we provide a comprehensive overview of the robotic technologies, the navigation methods, the dexterous functions and the translational challenges of wireless soft robotic medical devices. We first discuss safety and biocompatibility from a biological and technical perspective and then examine navigation methods for overcoming biological barriers for delivery, mobility and retrieval, highlighting dexterous medical functions at small scales. Finally, we identify key product development challenges, as well as the regulatory and ethical considerations that should be addressed to enable the clinical translation of wireless soft robotic medical devices.
Key points
-
Small-scale wireless soft robotic devices are promising for various clinical applications, but important challenges remain to be addressed to enable their clinical translation.
-
Proof-of-concept devices have been designed with various navigation and control strategies for overcoming biological barriers and for allowing device deployment, mobility and retrieval from the body.
-
Biocompatibility, navigation methods, basic and dexterous functions, and fabrication challenges should be solved interdependently with a holistic perspective.
-
Ethical concerns, regulatory requirements, scalable production techniques and financial sustainability should be addressed to enable the clinical translation of small-scale wireless soft robotic devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rus, D. & Tolley, M. T. Design, fabrication and control of soft robots. Nature 521, 467–475 (2015).
Ren, Z. & Sitti, M. Design and build of small-scale magnetic soft-bodied robots with multimodal locomotion. Nat. Protoc. https://doi.org/10.1038/s41596-023-00916-6 (2023). This article explains the key points for the design and fabrication of magnetic soft robots.
Wang, T. et al. Adaptive wireless millirobotic locomotion into distal vasculature. Nat. Commun. 13, 4465 (2022). This article describes an important example of a targeted biomedical application for wireless mobile soft robotic devices.
Hu, W., Lum, G. Z., Mastrangeli, M. & Sitti, M. Small-scale soft-bodied robot with multimodal locomotion. Nature 554, 81–85 (2018). This article describes the first wireless mobile soft millirobot with multimodal locomotion capability, inspired by soft-bodied small-scale animals.
Wu, S., Hu, W., Ze, Q., Sitti, M. & Zhao, R. Multifunctional magnetic soft composites: a review. Multifunct. Mater. 3, 042003 (2020).
Li, M. et al. Miniature coiled artificial muscle for wireless soft medical devices. Sci. Adv. 8, eabm5616 (2022).
Wang, C., Wu, Y., Dong, X., Armacki, M. & Sitti, M. In situ sensing physiological properties of biological tissues using wireless miniature soft robots. Sci. Adv. 9, eadg3988 (2023).
Soon, R. H. et al. On-demand anchoring of wireless soft miniature robots on soft surfaces. Proc. Natl Acad. Sci. USA 119, e2207767119 (2022).
Soon, R. H. et al. Pangolin-inspired untethered magnetic robot for on-demand biomedical heating applications. Nat. Commun. 14, 3320 (2023).
Dogan, N. O. et al. Remotely guided immunobots engaged in anti-tumorigenic phenotypes for targeted cancer immunotherapy. Small 18, e2204016 (2022).
Sridhar, V. et al. Designing covalent organic framework-based light-driven microswimmers towards therapeutic applications. Adv. Mater. 35, 2301126 (2023).
Noritsugu, T. Human-friendly soft actuator. Int. J. Jpn. Soc. Precis. Eng. 31, 92–96 (1997).
Miskin, M. Z. et al. Electronically integrated, mass-manufactured, microscopic robots. Nature 584, 557–561 (2020).
Abbott, J. et al. A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons. Nat. Biomed. Eng. 4, 232–241 (2020).
Iddan, G., Meron, G., Glukhovsky, A. & Swain, P. Wireless capsule endoscopy. Nature 405, 417 (2000).
Hermanson, G. T. Bioconjugate Techniques (Academic Press, 2013).
Li, M., Pal, A., Aghakhani, A., Pena-Francesch, A. & Sitti, M. Soft actuators for real-world applications. Nat. Rev. Mater. 7, 235–249 (2022).
Xia, Y. & Whitesides, G. M. Soft lithography. Angew. Chem. Int. Edn Engl. 37, 550–575 (1998).
Lendlein, A. & Kelch, S. Shape-memory polymers. Angew. Chem. Int. Edn Engl. 41, 2034–2057 (2002).
Maruo, S., Nakamura, O. & Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 22, 132–134 (1997).
Rogers, J. A., Someya, T. & Huang, Y. Materials and mechanics for stretchable electronics. Science 327, 1603–1607 (2010).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Sletten, E. M. & Bertozzi, C. R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 44, 666–676 (2011).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Sitti, M. Physical intelligence as a new paradigm. Extreme Mech. Lett. 46, 101340 (2021).
Levin, L. A. & Danesh-Meyer, H. V. Lost in translation: bumps in the road between bench and bedside. J. Am. Med. Assoc. 303, 1533–1534 (2010).
Hanscom, M. & Cave, D. R. Endoscopic capsule robot-based diagnosis, navigation and localization in the gastrointestinal tract. Front. Robot. AI 9, 896028 (2022).
Wang, L., Jiang, K. & Shen, G. Wearable, implantable, and interventional medical devices based on smart electronic skins. Adv. Mater. Technol. 6, 2100107 (2021).
Lam, R. H. W. & Chen, W. Biomedical Devices: Materials, Design, and Manufacturing (Springer, 2019).
Stavrinidou, E. & Proctor, C. M. Introduction to Bioelectronics: Materials, Devices, and Applications (AIP Publishing, 2022).
Wallin, R. F. & Arscott, E. F. A practical guide to ISO-10993-5: cytotoxicity. Med. Device Diagnostic Ind. 20, 96–98 (1998).
Strickland, J. et al. Status of acute systemic toxicity testing requirements and data uses by US regulatory agencies. Regul. Toxicol. Pharmacol. 94, 183–196 (2018).
Mohammadpour, R., Dobrovolskaia, M. A., Cheney, D. L., Greish, K. F. & Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug. Deliv. Rev. 144, 112–132 (2019).
Kabanov, A. V. Polymer genomics: an insight into pharmacology and toxicology of nanomedicines. Adv. Drug. Delivery Rev. 58, 1597–1621 (2006).
Bond, J. A. Review of the toxicology of styrene. Crit. Rev. Toxicol. 19, 227–249 (1989).
Chen, Q. & Thouas, G. A. Metallic implant biomaterials. Mater. Sci. Eng. R 87, 1–57 (2015).
Heise, T. et al. Report on investigation of ISO 10993-12 extraction conditions. Regul. Toxicol. Pharmacol. 131, 105164 (2022).
Majidi, C. Soft-matter engineering for soft robotics. Adv. Mater. Technol. 4, 1800477 (2019).
Mazzolai, B. et al. Roadmap on soft robotics: multifunctionality, adaptability and growth without borders. Multifunct. Mater. 5, 032001 (2022).
Coyle, S., Majidi, C., LeDuc, P. & Hsia, K. J. Bio-inspired soft robotics: material selection, actuation, and design. Extreme Mech. Lett. 22, 51–59 (2018).
Zhang, M. et al. Hydrogel muscles powering reconfigurable micro-metastructures with wide-spectrum programmability. Nat. Mater. 22, 1243–1252 (2023).
Hartmann, F., Baumgartner, M. & Kaltenbrunner, M. Becoming sustainable, the new frontier in soft robotics. Adv. Mater. 33, e2004413 (2021).
Cabanach, P. et al. Zwitterionic 3D-printed non-immunogenic stealth microrobots. Adv. Mater. 32, e2003013 (2020).
VanEpps, J. S. & Younger, J. G. Implantable device-related infection. Shock 46, 597–608 (2016).
Tipnis, N. P. & Burgess, D. J. Sterilization of implantable polymer-based medical devices: a review. Int. J. Pharm. 544, 455–460 (2018).
Zhang, D. et al. Dealing with the foreign-body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 31, 2007226 (2021).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Kizhakkedathu, J. N. & Conway, E. M. Biomaterial and cellular implants: foreign surfaces where immunity and coagulation meet. Blood 139, 1987–1998 (2022).
Serda, R. E. et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials 30, 2440–2448 (2009).
Franz, S., Rammelt, S., Scharnweber, D. & Simon, J. C. Immune responses to implants — a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692–6709 (2011).
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Harris, J. M., Martin, N. E. & Modi, M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 40, 539–551 (2001).
Williams, D. F. There is no such thing as a biocompatible material. Biomaterials 35, 10009–10014 (2014). This review takes a detailed look at biocompatibility tests for biomedical materials.
Cangelosi, A., Bongard, J., Fischer, M. H. & Nolfi, S. Embodied intelligence. In Springer Handbook of Computational Intelligence (eds Kacprzyk, J. & Pedrycz, W.) 697–714 (2015).
Zhang, J. et al. Voxelated three-dimensional miniature magnetic soft machines via multimaterial heterogeneous assembly. Sci. Robot. 6, eabf0112 (2021).
Utke, I. et al. Coordination and organometallic precursors of group 10 and 11: focused electron beam induced deposition of metals and insight gained from chemical vapour deposition, atomic layer deposition, and fundamental surface and gas phase studies. Coord. Chem. Rev. 458, 213851 (2022).
Barth, S., Huth, M. & Jungwirth, F. Precursors for direct-write nanofabrication with electrons. J. Mater. Chem. C 8, 15884–15919 (2020).
Baumgartner, M. et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 19, 1102–1109 (2020).
Kwok, S. W. et al. Magnetic assembly of soft robots with hard components. Adv. Funct. Mater. 24, 2180–2187 (2014).
Jonkheijm, P., van der Schoot, P., Schenning, A. P. & Meijer, E. W. Probing the solvent-assisted nucleation pathway in chemical self-assembly. Science 313, 80–83 (2006).
Daly, A. C., Prendergast, M. E., Hughes, A. J. & Burdick, J. A. Bioprinting for the biologist. Cell 184, 18–32 (2021).
Wendorff, J. H., Agarwal, S. & Greiner, A. Electrospinning: Materials, Processing, and Applications (John Wiley & Sons, 2012).
Hines, L., Petersen, K., Lum, G. Z. & Sitti, M. Soft actuators for small-scale robotics. Adv. Mater. 29, 1603483 (2017).
Sitti, M. Mobile Microrobotics (MIT Press, 2017).
Apsite, I., Salehi, S. & Ionov, L. Materials for smart soft actuator systems. Chem. Rev. 122, 1349–1415 (2022).
Wu, Y., Dong, X., Kim, J. K., Wang, C. & Sitti, M. Wireless soft millirobots for climbing three-dimensional surfaces in confined spaces. Sci. Adv. 8, eabn3431 (2022). This article demonstrates modelling, fabrication and control challenges for soft robotic devices on various surfaces of the gastrointestinal tract.
Magdanz, V., Stoychev, G., Ionov, L., Sanchez, S. & Schmidt, O. G. Stimuli-responsive microjets with reconfigurable shape. Angew. Chem. Int. Edn Engl. 53, 2673–2677 (2014).
Kagan, D. et al. Functionalized micromachines for selective and rapid isolation of nucleic acid targets from complex samples. Nano Lett. 11, 2083–2087 (2011).
Garcia-Gradilla, V. et al. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small 10, 4154–4159 (2014).
Esteban-Fernandez de Avila, B. et al. Single cell real-time miRNAs sensing based on nanomotors. ACS Nano 9, 6756–6764 (2015).
Kwon, G. H. et al. Biomimetic soft multifunctional miniature aquabots. Small 4, 2148–2153 (2008).
Scalet, G. Two-way and multiple-way shape memory polymers for soft robotics: an overview. Actuators 9, 10 (2020).
Akolpoglu, M. B. et al. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci. Adv. 8, eabo6163 (2022).
Palagi, S. et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 15, 647–653 (2016).
Kim, J. et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418 (2016).
Lee, G. H. et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 5, 149–165 (2020).
Sitti, M. et al. Biomedical applications of untethered mobile milli/microrobots. Proc. IEEE Inst. Electr. Electron. Eng. 103, 205–224 (2015).
Wang, B., Zhang, Y. & Zhang, L. Recent progress on micro- and nano-robots: towards in vivo tracking and localization. Quant. Imaging Med. Surg. 8, 461–479 (2018).
Son, D., Yim, S. & Sitti, M. A 5-D localization method for a magnetically manipulated untethered robot using a 2-D array of Hall-effect sensors. IEEE/ASME Trans. Mechatron. 21, 708–716 (2015).
Berkelman, P. & Abdul-Ghani, H. Electromagnetic haptic feedback system for use with a graphical display using flat coils and sensor array. IEEE Robot. Autom. Lett. 5, 1618–1625 (2020).
Wright, S. E., Mahoney, A. W., Popek, K. M. & Abbott, J. J. A spherical-magnet end-effector for robotic magnetic manipulation. In 2015 IEEE International Conference on Robotics and Automation (ICRA) 1190–1195 (IEEE, 2020).
Li, J., Esteban-Fernandez de Avila, B., Gao, W., Zhang, L. & Wang, J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci. Robot. 2, eaam6431 (2017).
Stanton, M. M. et al. Biohybrid microtube swimmers driven by single captured bacteria. Small 13, 1603679 (2017).
Miller, K. M., Mahoney, A. W., Schmid, T. & Abbott, J. J. Proprioceptive magnetic-field sensing for closed-loop control of magnetic capsule endoscopes. In 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems 1994–1999 (IEEE, 2012).
Kim, Y., Yuk, H., Zhao, R., Chester, S. A. & Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 558, 274–279 (2018).
Wang, X. et al. Untethered and ultrafast soft-bodied robots. Commun. Mater. 1, 67 (2020).
Song, S. et al. 6-D magnetic localization and orientation method for an annular magnet based on a closed-form analytical model. IEEE Trans. Magnetics 50, 5000411 (2014).
Lum, G. Z. et al. Shape-programmable magnetic soft matter. Proc. Natl Acad. Sci. USA 113, E6007–E6015 (2016).
Tiryaki, M. E., Demir, S. O. & Sitti, M. Deep learning-based 3D magnetic microrobot tracking using 2D MR images. IEEE Robot. Autom. Lett. 7, 6982–6989 (2022).
Abdallah, M. N. et al. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater. 54, 150–163 (2017).
Aghakhani, A. et al. High shear rate propulsion of acoustic microrobots in complex biological fluids. Sci. Adv. 8, eabm5126 (2022).
Nan, K. et al. Mucosa-interfacing electronics. Nat. Rev. Mater. 7, 908–925 (2022).
Mandsberg, N. K., Christfort, J. F., Kamguyan, K., Boisen, A. & Srivastava, S. K. Orally ingestible medical devices for gut engineering. Adv. Drug. Deliv. Rev. 165–166, 142–154 (2020).
Dong, X. et al. Bioinspired cilia arrays with programmable nonreciprocal motion and metachronal coordination. Sci. Adv. 6, eabc9323 (2020).
Bourquin, J. et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 30, e1704307 (2018).
Zhang, X. D. et al. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 33, 4628–4638 (2012).
Farah, S. et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat. Mater. 18, 892–904 (2019).
Cuttaz, E. et al. Conductive elastomer composites for fully polymeric, flexible bioelectronics. Biomater. Sci. 7, 1372–1385 (2019).
Xing, C. M. et al. Quantitative fabrication, performance optimization and comparison of PEG and zwitterionic polymer antifouling coatings. Acta Biomater. 59, 129–138 (2017).
Li, S. D. & Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 5, 496–504 (2008).
Mehta, D., Ravindran, K. & Kuebler, W. M. Novel regulators of endothelial barrier function. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L924–L935 (2014).
Rehmann, M. S. et al. Tuning and predicting mesh size and protein release from step growth hydrogels. Biomacromolecules 18, 3131–3142 (2017).
Thai, M. T. et al. Advanced soft robotic system for in situ 3D bioprinting and endoscopic surgery. Adv. Sci. 10, e2205656 (2023).
Loebel, C., Rodell, C. B., Chen, M. H. & Burdick, J. A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 12, 1521–1541 (2017).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Holohan, C., Van Schaeybroeck, S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Dill, K. A., Ghosh, K. & Schmit, J. D. Physical limits of cells and proteomes. Proc. Natl Acad. Sci. USA 108, 17876–17882 (2011).
Zhou, C. et al. Ferromagnetic soft catheter robots for minimally invasive bioprinting. Nat. Commun. 12, 5072 (2021).
Xiong, J., Chen, J. & Lee, P. S. Functional fibers and fabrics for soft robotics, wearables, and human–robot interface. Adv. Mater. 33, e2002640 (2021).
Ren, Z. et al. Soft-bodied adaptive multimodal locomotion strategies in fluid-filled confined spaces. Sci. Adv. 7, eabh2022 (2021). This article reports a crawling soft robot inside the body’s fluid-filled confined spaces.
Vagni, P. et al. POLYRETINA restores light responses in vivo in blind Göttingen minipigs. Nat. Commun. 13, 3678 (2022).
Kim, T. I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Choi, Y. S. et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 11, 5990 (2020).
Chen, J. C. et al. A wireless millimetric magnetoelectric implant for the endovascular stimulation of peripheral nerves. Nat. Biomed. Eng. 6, 706–716 (2022). This article reports an in vivo study showing a preclinical example of wireless millimetric robotic devices.
Nagarajan, N., Dupret-Bories, A., Karabulut, E., Zorlutuna, P. & Vrana, N. E. Enabling personalized implant and controllable biosystem development through 3D printing. Biotechnol. Adv. 36, 521–533 (2018).
Roche, E. T. et al. Soft robotic sleeve supports heart function. Sci. Transl. Med. 9, eaaf3925 (2017).
Son, D., Gilbert, H. & Sitti, M. Magnetically actuated soft capsule endoscope for fine-needle biopsy. Soft Robot. 7, 10–21 (2020).
Zheng, Z. et al. Electrodeposited superhydrophilic-superhydrophobic composites for untethered multi-stimuli-responsive soft millirobots. Adv. Sci. 10, e2302409 (2023).
Ren, Z., Hu, W., Dong, X. & Sitti, M. Multi-functional soft-bodied jellyfish-like swimming. Nat. Commun. 10, 2703 (2019).
Kashyap, V. et al. Multilayer fabrication of durable catheter-deployable soft robotic sensor arrays for efficient left atrial mapping. Sci. Adv. 6, eabc6800 (2020).
Go, G. et al. Multifunctional biodegradable microrobot with programmable morphology for biomedical applications. ACS Nano 15, 1059–1076 (2021).
Sitti, M. Miniature soft robots—road to the clinic. Nat. Rev. Mater. 3, 74–75 (2018).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021). This article demonstrates biodegradable soft sensors with wireless communication modality.
Boveiri, H. R., Khayami, R., Javidan, R. & Mehdizadeh, A. Medical image registration using deep neural networks: a comprehensive review. Comput. Electr. Eng. 87, 106767 (2020).
Lin, K., Li, Y., Sun, J., Zhou, D. & Zhang, Q. Multi-sensor fusion for body sensor network in medical human–robot interaction scenario. Inf. Fusion 57, 15–26 (2020).
Tiryaki, M. E., Elmacıoğlu, Y. G. & Sitti, M. Magnetic guidewire steering at ultrahigh magnetic fields. Sci. Adv. 9, eadg6438 (2023).
Cao, A., Dhanaliwala, A., Shi, J., Gade, T. P. & Park, B. J. Image-based marker tracking and registration for intraoperative 3D image-guided interventions using augmented reality. In Proc. SPIE Medical Imaging 2020 11318 (SPIE, 2020).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019). This article describes an example of in vivo testing of soft biocompatible materials with medical functions.
Tang, Y. et al. Wireless miniature magnetic phase-change soft actuators. Adv. Mater. 34, e2204185 (2022).
Mc Caffrey, C. et al. Continuum robotic caterpillar with wirelessly powered shape memory alloy actuators. Soft Robot. 7, 700–710 (2020).
Ebrahimi, N. et al. Magnetic actuation methods in bio/soft robotics. Adv. Funct. Mater. 31, 2005137 (2021).
Herbert, R., Lim, H. R., Rigo, B. & Yeo, W. H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 8, eabm1175 (2022).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Lei, I. M. et al. 3D printed biomimetic cochleae and machine learning co-modelling provides clinical informatics for cochlear implant patients. Nat. Commun. 12, 6260 (2021).
Zhang, C. et al. Wirelessly powered deformable electronic stent for noninvasive electrical stimulation of lower esophageal sphincter. Sci. Adv. 9, eade8622 (2023).
Iacovacci, V. et al. A fully implantable device for intraperitoneal drug delivery refilled by ingestible capsules. Sci. Robot. 6, eabh3328 (2021).
Jiang, L. et al. Flexible ultrasound-induced retinal stimulating piezo-arrays for biomimetic visual prostheses. Nat. Commun. 13, 3853 (2022).
De la Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Sharma, S. et al. Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics. Nat. Electron. 6, 242–256 (2023).
Ramadi, K. B. et al. Bioinspired, ingestible electroceutical capsules for hunger-regulating hormone modulation. Sci. Robot. 8, eade9676 (2023).
Yan, Y. et al. Magnetically assisted soft milli-tools for occluded lumen morphology detection. Sci. Adv. 9, eadi3979 (2023).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Wang, Q., Du, X., Jin, D. & Zhang, L. Real-time ultrasound Doppler tracking and autonomous navigation of a miniature helical robot for accelerating thrombolysis in dynamic blood flow. ACS Nano 16, 604–616 (2022).
Zhang, Z. et al. Treatment of ruptured and nonruptured aneurysms using a semisolid iodinated embolic agent. Adv. Mater. 34, e2108266 (2022).
Avery, R. K. et al. An injectable shear-thinning biomaterial for endovascular embolization. Sci. Transl. Med. 8, 365ra156 (2016).
Wang, T., Hu, W., Ren, Z. & Sitti, M. Ultrasound-guided wireless tubular robotic anchoring system. IEEE Robot. Autom. Lett. 5, 4859–4866 (2020).
Maier-Hauff, K. et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 103, 317–324 (2011).
Wang, W. et al. Cilia metasurfaces for electronically programmable microfluidic manipulation. Nature 605, 681–686 (2022).
Reynolds, M. F. et al. Microscopic robots with onboard digital control. Sci. Robot. 7, eabq2296 (2022). This article describes onboard computation capabilities in wireless devices.
Won, S. M., Cai, L., Gutruf, P. & Rogers, J. A. Wireless and battery-free technologies for neuroengineering. Nat. Biomed. Eng. 7, 405–423 (2023).
Feng, W. et al. Cholesteric liquid crystal polymeric coatings for colorful artificial muscles and motile humidity sensor skin integrated with magnetic composites. Adv. Funct. Mater. 33, 2300731 (2023).
Chen, S. et al. Biodegradable microrobots for DNA vaccine delivery. Adv. Healthc. Mater. 12, e2202921 (2023).
Diana, M. & Marescaux, J. Robotic surgery. Br. J. Surg. 102, e15–e28 (2015).
Moglia, A. et al. A systematic review of virtual reality simulators for robot-assisted surgery. Eur. Urol. 69, 1065–1080 (2016).
Erol, T., Mendi, A. F. & Doğan, D. The digital twin revolution in healthcare. In 4th Int. Symp. on Multidisciplinary Studies and Innovative Technologies (ISMSIT) https://doi.org/10.1109/ISMSIT50672.2020.9255249 (2020).
Croatti, A., Gabellini, M., Montagna, S. & Ricci, A. On the integration of agents and digital twins in healthcare. J. Med. Syst. 44, 161 (2020).
Felgner, S., Kocijancic, D., Frahm, M. & Weiss, S. Bacteria in cancer therapy: renaissance of an old concept. Int. J. Microbiol. 2016, 8451728 (2016).
Raman, R. & Langer, R. Biohybrid design gets personal: new materials for patient-specific therapy. Adv. Mater. 32, e1901969 (2020).
Lerner, M. B. et al. Hybrids of a genetically engineered antibody and a carbon nanotube transistor for detection of prostate cancer biomarkers. ACS Nano 6, 5143–5149 (2012).
Almeida, M. & Ranisch, R. Beyond safety: mapping the ethical debate on heritable genome editing interventions. Human. Soc. Sci. Commun. 9, 139 (2022).
Wrede, P., Aghakhani, A., Bozuyuk, U., Yildiz, E. & Sitti, M. Acoustic trapping and manipulation of hollow microparticles under fluid flow using a single-lens focused ultrasound transducer. ACS Appl. Mater. Interf. 15, 52224–52236 (2023).
Bozuyuk, U., Yildiz, E., Han, M., Demir, S. O. & Sitti, M. Size-dependent locomotion ability of surface microrollers on physiologically relevant microtopographical surfaces. Small 19, e2303396 (2023).
Sassoon, I. & Blanc, V. Antibody–drug conjugate (ADC) clinical pipeline: a review. Meth. Mol. Biol. 1045, 1–27 (2013).
Marei, H. E., Cenciarelli, C. & Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 22, 255 (2022).
Héder, M. From NASA to EU: the evolution of the TRL scale in public sector innovation. Innov. J. 22, 1–23 (2017).
Seva, R. R., Tan, A. L. S., Tejero, L. M. S. & Salvacion, M. L. D. S. Multi-dimensional readiness assessment of medical devices. Theor. Issues Ergon. Sci. 24, 189–205 (2023).
Lavin, A. et al. Technology readiness levels for machine learning systems. Nat. Commun. 13, 6039 (2022).
Schroeder, R. G., Linderman, K., Liedtke, C. & Choo, A. S. Six sigma: definition and underlying theory. J. Oper. Manag. 26, 536–554 (2007).
Rowe, P. G. Design Thinking (MIT Press, 1991).
Abras, C., Maloney-Krichmar, D. & Preece, J. User-centered design. In Encyclopedia of Human–Computer Interaction (ed. Bainbridge, W.) Vol. 37, 445–456 (Sage Publications, 2004).
Hede, S., Nunes, M. J. L., Ferreira, P. F. V. & Rocha, L. A. Incorporating sustainability in decision-making for medical device development. Technol. Soc. 35, 276–293 (2013).
Editorial. Traversing the valley of death. Nat. Rev. Bioeng. 1, 875 (2023).
Pya, Y. et al. First human use of a wireless coplanar energy transfer coupled with a continuous-flow left ventricular assist device. J. Heart Lung Transpl. 38, 339–343 (2019).
Tashi, T., Mbuya, V. B. & Gangadharappa, H. V. Corrective action and preventive actions and its importance in quality management system: a review. Int. J. Pharm. Qual. Assur. 7, 1–6 (2016).
Lunney, J. K. et al. Importance of the pig as a human biomedical model. Sci. Transl. Med. 13, eabd5758 (2021).
Ho, A. & Quick, O. Leaving patients to their own devices? Smart technology, safety and therapeutic relationships. BMC Med. Ethics 19, 18 (2018).
Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Bull. Med. Ethics 182, 17–23 (2002).
Kichloo, A. et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam. Med. Community Health 8, e000530 (2020).
Emanuel, E. J. & Emanuel, L. L. Four models of the physician–patient relationship. J. Am. Med. Assoc. 267, 2221–2226 (1992).
Kadakia, K. T., Dhruva, S. S., Caraballo, C., Ross, J. S. & Krumholz, H. M. Use of recalled devices in new device authorizations under the us food and drug administration’s 510(k) pathway and risk of subsequent recalls. J. Am. Med. Assoc. 329, 136–143 (2023).
Abuhav, I. ISO 13485: 2016: A Complete Guide to Quality Management in the Medical Device Industry (CRC Press, 2018).
Wu, Z., Lin, X., Zou, X., Sun, J. & He, Q. Biodegradable protein-based rockets for drug transportation and light-triggered release. ACS Appl. Mater. Interf. 7, 250–255 (2015).
Lee, Y. W. et al. Multifunctional 3D-printed pollen grain-inspired hydrogel microrobots for on-demand anchoring and cargo delivery. Adv. Mater. 35, e2209812 (2023).
Aydin, O. et al. Neuromuscular actuation of biohybrid motile bots. Proc. Natl Acad. Sci. USA 116, 19841–19847 (2019).
Ze, Q. et al. Spinning-enabled wireless amphibious origami millirobot. Nat. Commun. 13, 3118 (2022).
Wehner, M. et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 536, 451–455 (2016).
Jin, P. et al. A flexible, stretchable system for simultaneous acoustic energy transfer and communication. Sci. Adv. 7, eabg2507 (2021).
Dyro, J. (ed.). Clinical Engineering Handbook (Elsevier, 2004).
Tau, N. & Shepshelovich, D. Assessment of data sources that support US Food and Drug Administration medical devices safety communications. JAMA Intern. Med. 180, 1420–1426 (2020).
Everhart, A. O., Sen, S., Stern, A. D., Zhu, Y. & Karaca-Mandic, P. Association between regulatory submission characteristics and recalls of medical devices receiving 510 (k) clearance. J. Am. Med. Assoc. 329, 144–156 (2023).
Kramer, D. B. et al. Security and privacy qualities of medical devices: an analysis of FDA postmarket surveillance. PLoS One 7, e40200 (2012).
Hofer, M. P. et al. Regulatory watch: impact of scientific advice from the European Medicines Agency. Nat. Rev. Drug. Discov. 14, 302–303 (2015).
Flynn, R. et al. Marketing authorization applications made to the European Medicines Agency in 2018–2019: what was the contribution of real-world evidence? Clin. Pharmacol. Therapeut. 111, 90–97 (2022).
Cheng, M. Medical Device Regulations: Global Overview and Guiding Principles (World Health Organization, 2003).
Lamph, S. Regulation of medical devices outside the European Union. J. R. Soc. Med. 105, S12–S21 (2012).
Folefac, C. A. & Desmond, H. Clinical equipoise: why still the gold standard for randomized clinical trials? Clin. Ethics 17, 14777509221121107 (2022).
Zuckerman, D. M., Brown, P. & Nissen, S. E. Medical device recalls and the FDA approval process. Arch. Intern. Med. 171, 1006–1011 (2011).
Muth, C. C. Conflict of interest in medicine. J. Am. Med. Assoc. 317, 1812 (2017).
Baim, D. S. et al. Medical device development: managing conflicts of interest encountered by physicians. Catheter. Cardiovasc. Interv. 69, 655–664 (2007).
Acknowledgements
This work is funded by the Max Planck Society and the European Research Council (ERC) Advanced Grant (SoMMoR project, grant number 834531). Y.W. thanks the Alexander von Humboldt Foundation for financial support. E.Y. has received funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement number 101059593.
Author information
Authors and Affiliations
Contributions
T.W., Y.W. and E.Y. contributed equally to this work. M.S. initiated the Review, and all the authors developed its outline. All authors contributed to the writing and editing of the Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Huichan Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Wu, Y., Yildiz, E. et al. Clinical translation of wireless soft robotic medical devices. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00156-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00156-7