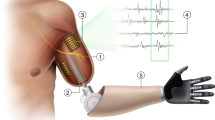
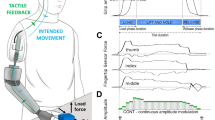
Abstract
Our expanding expertise in peripheral nerve regeneration and soft tissue reconstruction is enabling the development of novel innervated tissue constructs that can be combined with artificial interfacing technologies to facilitate control and sensation of limb prostheses. By utilizing the body’s native afferent and efferent signalling pathways, these mechanoneural interfaces have demonstrated the capacity to enhance volitional prosthetic control, refer somatosensory sensation within proprioceptive and cutaneous modalities, and reduce post-amputation pain. This Review discusses the biophysical principles underpinning recent advancements in targeted reinnervation techniques, regenerative peripheral nerve interfaces and agonist–antagonist neuromuscular architectures that can be combined with artificial technologies, including implanted electrodes, magnetic interfacing and osseointegrated structures, for improved integration with upper-extremity and lower-extremity prostheses. Expanding the capacity for bidirectional information transfer between the peripheral nervous system and external assistive devices will increase the potential of prosthetic embodiment and rehabilitation.
Key points
-
A mechanoneural interface combines surgically modified soft tissue constructs, such as nerves and muscles, with artificial components to enhance peripheral neural signalling for the reconstruction of bionic limbs
-
A more thorough understanding of end organ cross-innervation and regeneration dynamics may inform mechanoneural interface design
-
Mechanoneural interfaces afford improved physiological efferent and afferent neural signalling that could sustain prosthetic embodiment
-
Mechanoneural interface soft tissue constructs show reduced post-amputation pain and neuroma formation in clinical settings
-
Next-generation mechanoneural interfaces suggest additional utility with respect to efferent prosthetic control and prosthetic somatosensation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moghadasi, A. N. Artificial eye in burnt city and theoretical understanding of how vision works. Iran J. Public Health 43, 1595–1596 (2014).
Finch, J. L., Heath, G. H., David, A. R. & Kulkarni, J. Biomechanical assessment of two artificial big toe restorations from ancient Egypt and their significance to the history of prosthetics. J. Prosthet. Orthot. 24, 181–191 (2012).
Bhuyan, D. & Kumar, K. in Research Anthology on Emerging Technologies and Ethical Implications in Human Enhancement (ed. Khosrow-Pour, M.) 365–380 (IGI Global, 2021).
Weir, R. F., Heckathorne, C. W. & Childress, D. S. Cineplasty as a control input for externally powered prosthetic components. J. Rehabil. Res. Dev. 38, 357–363 (2001).
Kuiken, T. A. et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 301, 619–628 (2009).
Kung, T. A. et al. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast. Reconstr. Surg. 133, 1380–1394 (2014).
Clites, T. R. et al. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 10, eaap8373 (2018).
Herr, H. M. et al. Reinventing extremity amputation in the era of functional limb restoration. Ann. Surg. 273, 269–279 (2021).
Ehrsson, H. H. in Multisensory Perception (eds Sathian, K. & Ramachandran, V. S.) Ch. 8 (Academic Press, 2020).
Schofield, J. S. et al. Embodied cooperation to promote forgiving interactions with autonomous machines. Front. Neurorobot. 15, 661603 (2021).
Zbinden, J., Lendaro, E. & Ortiz-Catalan, M. Prosthetic embodiment: systematic review on definitions, measures, and experimental paradigms. J. Neuroeng. Rehabil. 19, 37 (2022).
Segil, J. L., Roldan, L. M. & Graczyk, E. L. Measuring embodiment: a review of methods for prosthetic devices. Front. Neurorobot. 16, 902162 (2022).
Azocar, A. F. et al. Design and clinical implementation of an open-source bionic leg. Nat. Biomed. Eng. 4, 941–953 (2020).
Carney, M. E., Shu, T., Stolyarov, R., Duval, J.-F. & Herr, H. M. Design and preliminary results of a reaction force series elastic actuator for bionic knee and ankle prostheses. IEEE Trans. Med. Robot. Bionics 3, 542–553 (2021).
Tropea, P., Mazzoni, A., Micera, S. & Corbo, M. Giuliano Vanghetti and the innovation of “cineplastic operations”. Neurology 89, 1627–1632 (2017).
Mazet, R. Cineplasty; historical review, present status, and critical evaluation of sixty-four patients. J. Bone Jt. Surg. Am. 40-A, 1389–1400 (1958).
Brav, E. A., Macdonald, W. F., Woodard, G. H. & Léonard, F. Follow-up notes on articles previously published in the journal. cineplasty — ten years later. J. Bone Jt. Surg. Am. 46, 1137–1138 (1964).
Kuiken, T. A., Dumanian, G. A., Lipschutz, R. D., Miller, L. A. & Stubblefield, K. A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 28, 245–253 (2004).
Vu, P. P. et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci. Transl. Med. 12, eaay2857 (2020).
Viton, J. M. et al. Equilibrium and movement control strategies in transtibial amputees. Prosthet. Orthot. Int. 24, 108–116 (2000).
Olenšek, A., Zadravec, M., Burger, H. & Matjačić, Z. Dynamic balancing responses in unilateral transtibial amputees following outward-directed perturbations during slow treadmill walking differ considerably for amputated and non-amputated side. J. Neuroeng. Rehabil. 18, 123 (2021).
Flor, H. et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375, 482–484 (1995).
Makin, T. R. et al. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 4, 1570 (2013).
Ortiz-Catalan, M. The stochastic entanglement and phantom motor execution hypotheses: a theoretical framework for the origin and treatment of phantom limb pain. Front. Neurol. 9, 748 (2018).
Wentink, E. C., Prinsen, E. C., Rietman, J. S. & Veltink, P. H. Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking. J. Neuroeng. Rehabil. 10, 87 (2013).
Andrysek, J. Lower-limb prosthetic technologies in the developing world: a review of literature from 1994–2010. Prosthet. Orthot. Int. 34, 378–398 (2010).
Zuo, K. J. & Olson, J. L. The evolution of functional hand replacement: from iron prostheses to hand transplantation. Plast. Surg. 22, 44–51 (2014).
Biddiss, E. & Chau, T. Upper-limb prosthetics: critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 86, 977–987 (2007).
Luza, L. P., Ferreira, E. G., Minsky, R. C., Pires, G. K. W. & da Silva, R. Psychosocial and physical adjustments and prosthesis satisfaction in amputees: a systematic review of observational studies. Disabil. Rehabil. Assist. Technol. 15, 582–589 (2020).
Safari, R. Lower limb prosthetic interfaces: clinical and technological advancement and potential future direction. Prosthet. Orthot. Int. 44, 384–401 (2020).
Murray, C. D. An interpretative phenomenological analysis of the embodiment of artificial limbs. Disabil. Rehabil. 26, 963–973 (2004).
Schaffalitzky, E., Gallagher, P., MacLachlan, M. & Wegener, S. T. Developing consensus on important factors associated with lower limb prosthetic prescription and use. Disabil. Rehabil. 34, 2085–2094 (2012).
Biddiss, E., Beaton, D. & Chau, T. Consumer design priorities for upper limb prosthetics. Disabil. Rehabil. Assist. Technol. 2, 346–357 (2007).
Cordella, F. et al. Literature review on needs of upper limb prosthesis users. Front. Neurosci. 10, 209 (2016).
Schaffalitzky, E., Gallagher, P., Maclachlan, M. & Ryall, N. Understanding the benefits of prosthetic prescription: exploring the experiences of practitioners and lower limb prosthetic users. Disabil. Rehabil. 33, 1314–1323 (2011).
Valle, G. et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45.e7 (2018).
Marasco, P. D. et al. Neurorobotic fusion of prosthetic touch, kinesthesia, and movement in bionic upper limbs promotes intrinsic brain behaviors. Sci. Robot. 6, eabf3368 (2021).
Bernshteĭn, N. A. The Co-ordination and Regulation of Movements (Pergamon Press, 1967).
Latash, M. L., Levin, M. F., Scholz, J. P. & Schöner, G. Motor control theories and their applications. Medicina 46, 382–392 (2010).
Haans, A., IJsselsteijn, W. A. & de Kort, Y. A. W. The effect of similarities in skin texture and hand shape on perceived ownership of a fake limb. Body Image 5, 389–394 (2008).
Rosén, B. et al. Referral of sensation to an advanced humanoid robotic hand prosthesis. Scand. J. Plast. Reconstr. Surg. Hand Surg. 43, 260–266 (2009).
Farmer, H., Tajadura-Jiménez, A. & Tsakiris, M. Beyond the colour of my skin: how skin colour affects the sense of body-ownership. Conscious. Cogn. 21, 1242–1256 (2012).
Zbinden, J., Lendaro, E. & Ortiz-Catalan, M. A multi-dimensional framework for prosthetic embodiment: a perspective for translational research. J. Neuroeng. Rehabil. 19, 122 (2022).
Makin, T. R., Holmes, N. P. & Ehrsson, H. H. On the other hand: dummy hands and peripersonal space. Behav. Brain Res. 191, 1–10 (2008).
Kalckert, A. & Ehrsson, H. H. Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front. Hum. Neurosci. 6, 40 (2012).
Farrer, C., Valentin, G. & Hupé, J. M. The time windows of the sense of agency. Conscious. Cogn. 22, 1431–1441 (2013).
Krugwasser, A. R., Harel, E. V. & Salomon, R. The boundaries of the self: the sense of agency across different sensorimotor aspects. J. Vis. 19, 14 (2019).
Antusch, S., Custers, R., Marien, H. & Aarts, H. Studying the sense of agency in the absence of motor movement: an investigation into temporal binding of tactile sensations and auditory effects. Exp. Brain Res. 239, 1795–1806 (2021).
Karsh, N., Eitam, B., Mark, I. & Higgins, E. T. Bootstrapping agency: how control-relevant information affects motivation. J. Exp. Psychol. Gen. 145, 1333–1350 (2016).
Gallagher, S. & Cole, J. Body image and body schema in a deafferented subject. J. Mind Behav. 16, 369–389 (1995).
Longo, M. R. in Perceptual and Emotional Embodiment (eds Coello, Y. & Fischer, M. H.) Ch. 6 (Routledge, 2015).
Cardinali, L. et al. Tool-use induces morphological updating of the body schema. Curr. Biol. 19, R478–R479 (2009).
De Preester, H. & Tsakiris, M. Body-extension versus body-incorporation: is there a need for a body-model? Phenomenol. Cogn. Sci. 8, 307–319 (2009).
Molina, C. S. & Faulk, J. Lower extremity amputation. In StatPearls (StatPearls Publishing, 2022).
Maduri, P. & Akhondi, H. Upper Limb Amputation. In StatPearls (StatPearls Publishing, 2023).
Tropf, J. G. & Potter, B. K. Osseointegration for amputees: current state of direct skeletal attachment of prostheses. Orthoplastic Surg. 12, 20–28 (2023).
Navarro, X. et al. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 10, 229–258 (2005).
Horch, K., Meek, S., Taylor, T. G. & Hutchinson, D. T. Object discrimination with an artificial hand using electrical stimulation of peripheral tactile and proprioceptive pathways with intrafascicular electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 483–489 (2011).
Davis, T. S. et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 13, 036001 (2016).
D’Anna, E. et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 4, eaau8892 (2019).
Dubin, A. E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Taylor, C. R. et al. Magnetomicrometry. Sci. Robot. 6, eabg0656 (2021).
McGlone, F. & Spence, C. The cutaneous senses: touch, temperature, pain/itch, and pleasure. Neurosci. Biobehav. Rev. 34, 145–147 (2010).
Proske, U. & Gandevia, S. C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697 (2012).
Augurelle, A.-S., Smith, A. M., Lejeune, T. & Thonnard, J.-L. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J. Neurophysiol. 89, 665–671 (2003).
Witney, A. G., Wing, A., Thonnard, J.-L. & Smith, A. M. The cutaneous contribution to adaptive precision grip. Trends Neurosci. 27, 637–643 (2004).
Libouton, X., Barbier, O., Berger, Y., Plaghki, L. & Thonnard, J.-L. Tactile roughness discrimination of the finger pad relies primarily on vibration sensitive afferents not necessarily located in the hand. Behav. Brain Res. 229, 273–279 (2012).
Weiler, J., Gribble, P. L. & Pruszynski, J. A. Spinal stretch reflexes support efficient control of reaching. J. Neurophysiol. 125, 1339–1347 (2021).
Johnson, K. O., Yoshioka, T. & Vega–Bermudez, F. Tactile functions of mechanoreceptive afferents innervating the hand. J. Clin. Neurophysiol. 17, 539–558 (2000).
Yau, J. M., Kim, S. S., Thakur, P. H. & Bensmaia, S. J. Feeling form: the neural basis of haptic shape perception. J. Neurophysiol. 115, 631–642 (2016).
Ebied, A. M., Kemp, G. J. & Frostick, S. P. The role of cutaneous sensation in the motor function of the hand. J. Orthop. Res. 22, 862–866 (2004).
Voisin, J., Lamarre, Y. & Chapman, C. E. Haptic discrimination of object shape in humans: contribution of cutaneous and proprioceptive inputs. Exp. Brain Res. 145, 251–260 (2002).
Giachritsis, C., Bevins, R. & Wing, A. The contribution of proprioceptive and cutaneous cues in weight perception: early evidence for maximum-likelihood integration. in Haptics: Generating and Perceiving Tangible Sensations. EuroHaptics 2010. Lecture Notes in Computer Science vol. 6191 (eds Kappers, A. M. L., van Erp, J. B. F., Bergmann Tiest, W. M. & van der Helm, F. C. T.) 11–16 (Springer, 2010).
Meyer, P. F., Oddsson, L. I. E. & De Luca, C. J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 156, 505–512 (2004).
Eils, E. et al. Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20, 54–60 (2004).
Höhne, A., Ali, S., Stark, C. & Brüggemann, G.-P. Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. Eur. J. Appl. Physiol. 112, 3829–3838 (2012).
Viseux, F. et al. How can the stimulation of plantar cutaneous receptors improve postural control? Review and clinical commentary. Neurophysiol. Clin. 49, 263–268 (2019).
Dietz, V. Proprioception and locomotor disorders. Nat. Rev. Neurosci. 3, 781–790 (2002).
Zehr, E. P. & Stein, R. B. What functions do reflexes serve during human locomotion? Prog. Neurobiol. 58, 185–205 (1999).
Navarro, X., Verdú, E. & Butí, M. Comparison of regenerative and reinnervating capabilities of different functional types of nerve fibers. Exp. Neurol. 129, 217–224 (1994).
Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 21, 8652 (2020).
Brushart, T. M. Preferential motor reinnervation: a sequential double-labeling study. Restor. Neurol. Neurosci. 1, 281–287 (1990).
Brushart, T. M. Motor axons preferentially reinnervate motor pathways. J. Neurosci. 13, 2730–2738 (1993).
Brushart, T. M., Gerber, J., Kessens, P., Chen, Y.-G. & Royall, R. M. Contributions of pathway and neuron to preferential motor reinnervation. J. Neurosci. 18, 8674–8681 (1998).
Bolívar, S. & Udina, E. Preferential regeneration and collateral dynamics of motor and sensory neurons after nerve injury in mice. Exp. Neurol. 358, 114227 (2022).
Sulaiman, W. & Gordon, T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J. 13, 100–108 (2013).
Scott, B. B., Winograd, J. M. & Redmond, R. W. Surgical approaches for prevention of neuroma at time of peripheral nerve injury. Front. Surg. 9, 819608 (2022).
Pet, M. A., Ko, J. H., Friedly, J. L., Mourad, P. D. & Smith, D. G. Does targeted nerve implantation reduce neuroma pain in amputees? Clin. Orthop. Relat. Res. 472, 2991–3001 (2014).
Dumanian, G. A. et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann. Surg. 270, 238–246 (2019).
Adidharma, W. et al. Sensory nerve regeneration and reinnervation in muscle following peripheral nerve injury. Muscle Nerve 66, 384–396 (2022).
Hooper, R. C. et al. Regenerative peripheral nerve interfaces for the management of symptomatic hand and digital neuromas. Plast. Reconstr. Surg. Glob. Open 8, e2792 (2020).
Buchheit, T. et al. Pain phenotypes and associated clinical risk factors following traumatic amputation: results from veterans integrated pain evaluation research (VIPER). Pain Med. 17, 149–161 (2016).
Buch, N. S., Qerama, E., Brix Finnerup, N. & Nikolajsen, L. Neuromas and postamputation pain. Pain 161, 147 (2020).
Svientek, S. R., Kemp, S. W. P., Cederna, P. S. & Kung, T. A. The clinical significance of a swollen neuroma: a meaningful distinction or an incidental finding? Ann. Palliat. Med. 9, 4412–4415 (2020).
Hsu, E. & Cohen, S. P. Postamputation pain: epidemiology, mechanisms, and treatment. J. Pain Res. 6, 121–136 (2013).
Bensmaia, S. J., Tyler, D. J. & Micera, S. Restoration of sensory information via bionic hands. Nat. Biomed. Eng. 7, 443–455 (2020).
Kim, K. A review of haptic feedback through peripheral nerve stimulation for upper extremity prosthetics. Curr. Opin. Biomed. Eng. 21, 100368 (2022).
Zollo, L. et al. Restoring tactile sensations via neural interfaces for real-time force-and-slippage closed-loop control of bionic hands. Sci. Robot. 4, eaau9924 (2019).
George, J. A. et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 4, eaax2352 (2019).
McCreery, D. B., Yuen, T. G. H., Agnew, W. F. & Bullara, L. A. A characterization of the effects on neuronal excitability due to prolonged microstimulation with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 44, 931–939 (1997).
Günter, C., Delbeke, J. & Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. J. Neuroeng. Rehabil. 16, 13 (2019).
Sensinger, J. W. & Dosen, S. A review of sensory feedback in upper-limb prostheses from the perspective of human motor control. Front. Neurosci. 14, 345 (2020).
D’Anna, E. et al. A somatotopic bidirectional hand prosthesis with transcutaneous electrical nerve stimulation based sensory feedback. Sci. Rep. 7, 10930 (2017).
Wendelken, S. et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14, 121 (2017).
Segil, J. L., Cuberovic, I., Graczyk, E. L., Weir, R. F. F. & Tyler, D. Combination of simultaneous artificial sensory percepts to identify prosthetic hand postures: a case study. Sci. Rep. 10, 6576 (2020).
Page, D. M. et al. Discriminability of multiple cutaneous and proprioceptive hand percepts evoked by intraneural stimulation with Utah slanted electrode arrays in human amputees. J. Neuroeng. Rehabil. 18, 12 (2021).
Petrini, F. M. et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 11, eaav8939 (2019).
Kuiken, T. A., Marasco, P. D., Lock, B. A., Harden, R. N. & Dewald, J. P. A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl Acad. Sci. USA 104, 20061–20066 (2007).
Hebert, J. S. et al. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 22, 765–773 (2014).
Hebert, J. S., Chan, K. M. & Dawson, M. R. Cutaneous sensory outcomes from three transhumeral targeted reinnervation cases. Prosthet. Orthot. Int. 40, 303–310 (2016).
Marasco, P. D. et al. Illusory movement perception improves motor control for prosthetic hands. Sci. Transl. Med. 10, eaao6990 (2018).
Vu, P. P. et al. Restoration of proprioceptive and cutaneous sensation using regenerative peripheral nerve interfaces in humans with upper limb amputations. Plast. Reconstr. Surg. 149, 1149e–1154e (2022).
Clites, T. R., Herr, H. M., Srinivasan, S. S., Zorzos, A. N. & Carty, M. J. The Ewing amputation: the first human implementation of the agonist-antagonist myoneural interface. Plast. Reconstr. Surg. Glob. Open 6, e1997 (2018).
Srinivasan, S. S. et al. Agonist-antagonist myoneural interface amputation preserves proprioceptive sensorimotor neurophysiology in lower limbs. Sci. Transl. Med. 12, eabc5926 (2020).
Song, H. et al. Agonist-antagonist muscle strain in the residual limb preserves motor control and perception after amputation. Commun. Med. 2, 97 (2022).
Srinivasan, S. S. et al. Agonist-antagonist myoneural interfaces in above-knee amputation preserve distal joint function and perception. Ann. Surg. 273, e115–e118 (2021).
Rijnbeek, E. H., Eleveld, N. & Olthuis, W. Update on peripheral nerve electrodes for closed-loop neuroprosthetics. Front. Neurosci. 12, 350 (2018).
Rossini, P. M. et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin. Neurophysiol. 121, 777–783 (2010).
Ngan, C. G. Y., Kapsa, R. M. I. & Choong, P. F. M. Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing. Materials 12, 1927 (2019).
Warren, D. J. et al. Recording and decoding for neural prostheses. Proc. IEEE 104, 374–391 (2016).
Konsten, J. et al. Comparison of epineural or intramuscular nerve electrodes for stimulated graciloplasty. Dis. Colon Rectum 44, 581–586 (2001).
Ghafoor, U., Kim, S. & Hong, K.-S. Selectivity and longevity of peripheral-nerve and machine interfaces: a review. Front. Neurorobot. 11, 59 (2017).
George, J. A. et al. Long-term performance of Utah slanted electrode arrays and intramuscular electromyographic leads implanted chronically in human arm nerves and muscles. J. Neural Eng. 17, 056042 (2020).
Grill, W. M. & Mortimer, J. T. Neural and connective tissue response to long-term implantation of multiple contact nerve cuff electrodes. J. Biomed. Mater. Res. 50, 215–226 (2000).
Branner, A., Stein, R. B., Fernandez, E., Aoyagi, Y. & Normann, R. A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 51, 146–157 (2004).
Renz, A. F., Reichmuth, A. M., Stauffer, F., Thompson-Steckel, G. & Vörös, J. A guide towards long-term functional electrodes interfacing neuronal tissue. J. Neural Eng. 15, 061001 (2018).
Kagan, Z. B. et al. Linear methods for reducing EMG contamination in peripheral nerve motor decodes. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 3422–3425 (2016).
Popovic, D. B. et al. Sensory nerve recording for closed-loop control to restore motor functions. IEEE Trans. Biomed. Eng. 40, 1024–1031 (1993).
Zbinden, J. et al. Improved control of a prosthetic limb by surgically creating electro-neuromuscular constructs with implanted electrodes. Sci. Transl. Med. 15, eabq3665 (2023).
Vu, P. P. et al. Long-term upper-extremity prosthetic control using regenerative peripheral nerve interfaces and implanted EMG electrodes. J. Neural Eng. 20, 026039 (2023).
Cheesborough, J. E., Smith, L. H., Kuiken, T. A. & Dumanian, G. A. Targeted muscle reinnervation and advanced prosthetic arms. Semin. Plast. Surg. 29, 62–72 (2015).
Hargrove, L. J. et al. Robotic leg control with EMG decoding in an amputee with nerve transfers. N. Engl. J. Med. 369, 1237–1242 (2013).
Kuiken, T. A. et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet 369, 371–380 (2007).
Pierrie, S. N., Gaston, R. G. & Loeffler, B. J. Targeted muscle reinnervation for prosthesis optimization and neuroma management in the setting of transradial amputation. J. Hand Surg. Am. 44, 525.e1–525.e8 (2019).
Kubiak, C. A., Kemp, S. W. P., Cederna, P. S. & Kung, T. A. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast. Reconstr. Surg. 144, 421e–430e (2019).
Di Valerio, E. et al. Efficacy of targeted muscle reinnervation for treating and preventing postamputation pain — a systematic review. Plast. Aesthet. Res. 9, 62 (2022).
Henderson, J. T., Koenig, Z. A., Climov, M. & Gelman, J. Demystifying targeted muscle reinnervation: a systematic review of nerve transfers for the lower extremity. Plast. Reconstr. Surg. Glob. Open 11, e4894 (2023).
Kuiken, T. A., Barlow, A. K., Hargrove, L. J. & Dumanian, G. A. Targeted muscle reinnervation for the upper and lower extremity. Tech. Orthop. 32, 109–116 (2017).
Peters, B. R., Russo, S. A., West, J. M., Moore, A. M. & Schulz, S. A. Targeted muscle reinnervation for the management of pain in the setting of major limb amputation. SAGE Open Med. 8, 205031212095918 (2020).
Hargrove, L. J., Miller, L. A., Turner, K. & Kuiken, T. A. Myoelectric pattern recognition outperforms direct control for transhumeral amputees with targeted muscle reinnervation: a randomized clinical trial. Sci. Rep. 7, 13840 (2017).
Hargrove, L., Miller, L., Turner, K. & Kuiken, T. Control within a virtual environment is correlated to functional outcomes when using a physical prosthesis. J. Neuroeng. Rehabil. 15, 60 (2018).
Simon, A. M. et al. Myoelectric prosthesis hand grasp control following targeted muscle reinnervation in individuals with transradial amputation. PLoS ONE 18, e0280210 (2023).
Mioton, L. M. et al. Targeted muscle reinnervation improves residual limb pain, phantom limb pain, and limb function: a prospective study of 33 major limb amputees. Clin. Orthop. Relat. Res. 478, 2161–2167 (2020).
Salminger, S. et al. Outcomes, challenges, and pitfalls after targeted muscle reinnervation in high-level amputees: is it worth the effort? Plast. Reconstr. Surg. 144, 1037e–1043e (2019).
Hebert, J. S., Elzinga, K., Chan, K. M., Olson, J. & Morhart, M. Updates in targeted sensory reinnervation for upper limb amputation. Curr. Surg. Rep. 2, 45 (2014).
Dumanian, G. A. et al. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast. Reconstr. Surg. 124, 863–869 (2009).
Souza, J. M. et al. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin. Orthop. Relat. Res. 472, 2984–2990 (2014).
Felder, J. M. et al. Failed targeted muscle reinnervation: findings at revision surgery and concepts for success. Plast. Reconstr. Surg. Glob. Open 10, e4229 (2022).
Bowen, J. B., Ruter, D., Wee, C., West, J. & Valerio, I. L. Targeted muscle reinnervation technique in below-knee amputation. Plast. Reconstr. Surg. 143, 309–312 (2019).
Schofield, J. S., Shell, C. E., Beckler, D. T., Thumser, Z. C. & Marasco, P. D. Long-term home-use of sensory-motor-integrated bidirectional bionic prosthetic arms promotes functional, perceptual, and cognitive changes. Front. Neurosci. 14, 120 (2020).
Serino, A. et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 140, 2993–3011 (2017).
Gardetto, A. et al. Reduction of phantom limb pain and improved proprioception through a TSR-based surgical technique: a case series of four patients with lower limb amputation. J. Clin. Med. 10, 4029 (2021).
Marasco, P. D., Kim, K., Colgate, J. E., Peshkin, M. A. & Kuiken, T. A. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain 134, 747–758 (2011).
Wells, M. R., Vaidya, U., Ricci, J. L. & Christie, C. A neuromuscular platform to extract electrophysiological signals from lesioned nerves: a technical note. J. Rehabil. Res. Dev. 38, 385–390 (2001).
Baldwin, J., Moon, J., Cederna, P. & Urbanchek, M. Early muscle revascularization and regeneration at the regenerative peripheral nerve interface. Plast. Reconstr. Surg. 130, abstr. 99 (2012).
Leach, G. A. et al. Regenerative peripheral nerve interface surgery: anatomic and technical guide. Plast. Reconstr. Surg. Glob. Open 11, e5127 (2023).
von Guionneau, N. et al. Vascularized Denervated Muscle Targets Deliver High Quality Signal Amplification. ASPN Nerve Week https://meeting.peripheralnerve.org/abstracts/2021/P13.cgi (2021).
Valerio, I., Schulz, S. A., West, J., Westenberg, R. F. & Eberlin, K. R. Targeted muscle reinnervation combined with a vascularized pedicled regenerative peripheral nerve interface. Plast. Reconstr. Surg. Glob. Open 8, e2689 (2020).
Herr, H. M., Riso, R. R., Song, K. W. Jr, Casler, R. J. & Carty, M. J. Peripheral neural interface via nerve regeneration to distal tissues. US patent 20150173918A1 (2015).
Srinivasan, S. S. et al. On prosthetic control: a regenerative agonist-antagonist myoneural interface. Sci. Robot. 2, eaan2971 (2017).
Clites, T. R., Carty, M. J., Srinivasan, S., Zorzos, A. N. & Herr, H. M. A murine model of a novel surgical architecture for proprioceptive muscle feedback and its potential application to control of advanced limb prostheses. J. Neural Eng. 14, 036002 (2017).
Herr, H. M. et al. Method and system for providing proprioceptive feedback and functionality mitigating limb pathology. US patent 20190021883A1 (2019).
Srinivasan, S. S., Diaz, M., Carty, M. & Herr, H. M. Towards functional restoration for persons with limb amputation: a dual-stage implementation of regenerative agonist-antagonist myoneural interfaces. Sci. Rep. 9, 1981 (2019).
Clites, T. R. et al. Caprine models of the agonist-antagonist myoneural interface implemented at the above- and below-knee amputation levels. Plast. Reconstr. Surg. 144, 218e–229e (2019).
Farahat, W. A. & Herr, H. M. Optimal workloop energetics of muscle-actuated systems: an impedance matching view. PLoS Comput. Biol. 6, e1000795 (2010).
Shu, T. et al. Modulation of prosthetic ankle plantarflexion through direct myoelectric control of a subject-optimized neuromuscular model. IEEE Robot. Autom. Lett. 7, 7620–7627 (2022).
Srinivasan, S. S. et al. Neural interfacing architecture enables enhanced motor control and residual limb functionality postamputation. Proc. Natl Acad. Sci. USA 118, e2019555118 (2021).
Herr, H. & Carty, M. J. The agonist-antagonist myoneural interface. Tech. Orthop. 36, 337–344 (2021).
Kubiak, C. A. et al. Physiologic signaling and viability of the muscle cuff regenerative peripheral nerve interface (MC-RPNI) for intact peripheral nerves. J. Neural Eng. 18, 0460d5 (2021).
Svientek, S. R. et al. The muscle cuff regenerative peripheral nerve interface for the amplification of intact peripheral nerve signals. J. Vis. Exp. 179, e63222 (2022).
Svientek, S. R. et al. QS7: physiologic signaling of the muscle cuff regenerative peripheral nerve interface (MC-RPNI) during volitional behavior. Plast. Reconstr. Surg. Glob. Open 9, 19–20 (2021).
Svientek, S. R., Ursu, D. C., Cederna, P. S. & Kemp, S. W. P. Fabrication of the composite regenerative peripheral nerve interface (C-RPNI) in the adult rat. J. Vis. Exp. 156, e60841 (2020).
Svientek, S., Dehdashtian, A., Bratley, J., Cederna, P. S. & Kemp, S. W P. Surface-level regenerative peripheral nerve interfaces (RPNIs) for a novel control method of advanced prosthetic devices. ASPN Nerve Week https://meeting.peripheralnerve.org/abstracts/2021/P2.cgi (2021).
Sando, I. C. et al. Dermal-based peripheral nerve interface for transduction of sensory feedback. Plast. Reconstr. Surg. 136, 19–20 (2015).
Sando, I. C. et al. Dermal sensory regenerative peripheral nerve interface (DS-RPNI) for re-establishing sensory nerve feedback in peripheral afferents in the rat. Plast. Reconstr. Surg. 151, 804e–813e (2022).
Lee, J. C., Adidharma, W., Dehdashtian, A., Cederna, P. S. & Kemp, S. W. QS35. Dermal sensory regenerative peripheral nerve interface (DS-RPNI) for multimodal sensory feedback. Plast. Reconstr. Surg. Glob. Open 10, 112 (2022).
Calotta, N. A., Hanwright, P. J., Giladi, A. & Tuffaha, S. H. Vascularized, denervated muscle targets for treatment of symptomatic neuromas in the upper extremity: description of operative technique. Tech. Hand Up. Extrem. Surg. 26, 141–145 (2022).
Tuffaha, S. H. et al. Vascularized, denervated muscle targets: a novel approach to treat and prevent symptomatic neuromas. Plast. Reconstr. Surg. Glob. Open 8, e2779 (2020).
Suresh, V., Schaefer, E. J., Calotta, N. A., Giladi, A. M. & Tuffaha, S. H. Use of vascularized, denervated muscle targets for prevention and treatment of upper-extremity neuromas. J. Hand Surg. Glob. Online 5, 92–96 (2022).
Woo, S. L. et al. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast. Reconstr. Surg. Glob. Open 4, e1038 (2016).
Burke, K. L., Kung, T. A., Hooper, R. C., Kemp, S. W. P. & Cederna, P. S. Regenerative peripheral nerve interfaces (RPNIs): current status and future direction. Plast. Aesthet. Res. 9, 48 (2022).
Larson, J. V. et al. Prototype sensory regenerative peripheral nerve interface for artificial limb somatosensory feedback. Plast. Reconstr. Surg. 133, abstr. 17 (2014).
Srinivasan, S. & Herr, H. M. A cutaneous mechanoneural interface for neuroprosthetic feedback. Nat. Biomed. Eng. 6, 731–740 (2022).
Herr, H., Song, H. & Srinivasan, S. Mechanoneural interfaces for prosthetic control. US patent 20230050411A1 (2023).
Cederna, P., Nghiem, B., Hu, Y., Sando, I. & Urbanchek, M. Sensory protection to enhance functional recovery following proximal nerve injuries: current trends. Plast. Aesthet. Res. 2, 202 (2015).
Li, G. et al. in Neural Interface: Frontiers and Applications (ed. Zheng, X.) 149–166 (Springer, 2019).
Samuel, O. W. et al. Intelligent EMG pattern recognition control method for upper-limb multifunctional prostheses: advances, current challenges, and future prospects. IEEE Access 7, 10150–10165 (2019).
Ortiz-Catalan, M., Håkansson, B. & Brånemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 6, 257re6 (2014).
Tarantino, S., Clemente, F., Barone, D., Controzzi, M. & Cipriani, C. The myokinetic control interface: tracking implanted magnets as a means for prosthetic control. Sci. Rep. 7, 17149 (2017).
Moradi, A. et al. Clinical implementation of a bionic hand controlled with kineticomyographic signals. Sci. Rep. 12, 14805 (2022).
Hettiarachchi, N., Ju, Z. & Liu, H. A new wearable ultrasound muscle activity sensing system for dexterous prosthetic control. in IEEE International Conference on Systems, Man, and Cybernetics (ed. O'Conner, L.) 1415–1420 (IEEE, 2015).
Akhlaghi, N. et al. Real-time classification of hand motions using ultrasound imaging of forearm muscles. IEEE Trans. Biomed. Eng. 63, 1687–1698 (2016).
Hartmann, C., Došen, S., Amsuess, S. & Farina, D. Closed-loop control of myoelectric prostheses with electrotactile feedback: influence of stimulation artifact and blanking. IEEE Trans. Neural Syst. Rehabil. Eng. 23, 807–816 (2015).
Srinivasan, S. S., Maimon, B. E., Diaz, M., Song, H. & Herr, H. M. Closed-loop functional optogenetic stimulation. Nat. Commun. 9, 5303 (2018).
Montero, J., Clemente, F. & Cipriani, C. Feasibility of generating 90 Hz vibrations in remote implanted magnets. Sci. Rep. 11, 15456 (2021).
Maimon, B. E. et al. Optogenetic peripheral nerve immunogenicity. Sci. Rep. 8, 14076 (2018).
Fleming, A. et al. Myoelectric control of robotic lower limb prostheses: a review of electromyography interfaces, control paradigms, challenges and future directions. J. Neural Eng. 18, 041004 (2021).
Das, S. et al. Innervation: the missing link for biofabricated tissues and organs. NPJ Regen. Med. 5, 11 (2020).
Münger, M. et al. Protective and risk factors for phantom limb pain and residual limb pain severity. Pain Pract. 20, 578–587 (2020).
Shu, T., Huang, S. S., Shallal, C. & Herr, H. M. Restoration of bilateral motor coordination from preserved agonist-antagonist coupling in amputation musculature. J. Neuroeng. Rehabil. 18, 38 (2021).
Acknowledgements
This work was funded by the K. Lisa Yang Center for Bionics at the Massachusetts Institute of Technology.
Author information
Authors and Affiliations
Contributions
T.S. and G.H. researched data, contributed to content discussion, wrote the manuscript and contributed equally. C.T. researched data, contributed to content discussion and wrote the manuscript. H.H. contributed to content discussion and wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
H.H. holds patents on AMI and CMI mechanoneural interfacing technologies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Paul Marasco and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shu, T., Herrera-Arcos, G., Taylor, C.R. et al. Mechanoneural interfaces for bionic integration. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00151-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00151-y