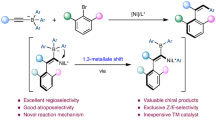
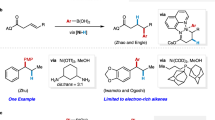
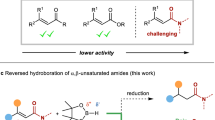
Abstract
Enantioenriched boronic esters are widely used multipurpose building blocks in organic synthesis. Multicomponent processes that deliver these organoboron compounds using non-precious single-catalyst systems are highly sought-after but remain rare. This owes to the lack of an appropriate chiral ligand that is capable of inducing high efficiency and selectivity. Here we report the use of an Ni-based catalyst containing a chiral N-heterocyclic carbene for the enantioselective 1,2-carboboration of unactivated and activated alkenes without the need for directing groups. Various aryl and alkenyl motifs can be successfully installed to afford functionalized alkylboronates bearing tertiary or quaternary β-stereocentres with good to excellent regio- and enantioselectivity. Contrary to previously reported carboboration reactions, mechanistic studies suggest that the reported Ni-catalysed transformation proceeds through a regio- and stereo-determining carbonickelation and subsequent borylation. The utility of the approach is demonstrated by the concise synthesis of key biologically active molecules.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information.
References
Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials 2nd ed. 1–109 (Wiley-VCH, 2011).
Yeung, K., Mykura, R. C. & Aggarwal, V. K. Lithiation–borylation methodology in the total synthesis of natural products. Nat. Synth. 1, 117–126 (2022).
Matteson, D. S. Boronic esters in asymmetric synthesis. J. Org. Chem. 78, 10009–10023 (2013).
Andrés, P., Ballano, G., Calaza, M. I. & Cativiela, C. Synthesis of α-aminoboronic acids. Chem. Soc. Rev. 45, 2291–2307 (2016).
Jäkle, F. Recent advances in the synthesis and applications of organoborane polymers. in Synthesis and Application of Organoboron Compounds (eds Fernández, E. & Whiting, A.) 297–325 (Springer International Publishing, 2015).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Aiken, S. G., Bateman, J. M. & Aggarwal, V. K. Boron “ate” complexes for asymmetric synthesis. in Science of Synthesis: Advances in Organoboron Chemistry towards Organic Synthesis Ch. 13, 393–458 (Thieme, 2019).
Zhang, C., Hu, W. & Morken, J. P. α-Boryl organometallic reagents in catalytic asymmetric synthesis. ACS Catal. 11, 10660–10680 (2021).
Xu, N., Liang, H. & Morken, J. P. Copper-catalyzed stereospecific transformations of alkylboronic esters. J. Am. Chem. Soc. 144, 11546–11552 (2022).
Wang, M. & Shi, Z. Methodologies and strategies for selective borylation of C–Het and C–C bonds. Chem. Rev. 120, 7348–7398 (2020).
Collins, B. S. L., Wilson, C. M., Myers, E. L. & Aggarwal, V. K. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 56, 11700–11733 (2017).
Whyte, A., Torelli, A., Mirabi, B., Zhang, A. & Lautens, M. Copper-catalyzed borylative difunctionalization of π-systems. ACS Catal. 10, 11578–11622 (2020).
Hu, J., Ferger, M., Shi, Z. & Marder, T. B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 50, 13129–13188 (2021).
Ye, Y. et al. Nickel-catalyzed enantioselective 1,2-vinylboration of styrenes. Chem. Sci. 12, 13209–13215 (2021).
Bergmann, A. M., Dorn, S. K., Smith, K. B., Logan, K. M. & Brown, M. K. Catalyst-controlled 1,2- and 1,1-arylboration of α-alkyl alkenyl arenes. Angew. Chem. Int. Ed. 58, 1719–1723 (2019).
Jia, T., Cao, P., Wang, B., Lou, Y. & Liao, J. A Cu/Pd cooperative catalysis for enantioselective allylboration of alkenes. J. Am. Chem. Soc. 137, 13760–13763 (2015).
Dorn, S. K. & Brown, M. K. Cooperative Pd/Cu catalysis for alkene arylboration: opportunities for divergent reactivity. ACS Catal. 12, 2058–2063 (2022).
Lee, H., Lee, S. & Yun, J. Pd-catalyzed stereospecific cross-coupling of chiral α-borylalkylcopper species with aryl bromides. ACS Catal. 10, 2069–2073 (2020).
Chen, B. et al. Modular synthesis of enantioenriched 1,1,2-triarylethanes by an enantioselective arylboration and cross-coupling sequence. ACS Catal. 7, 2425–2429 (2017).
Chen, B., Cao, P., Liao, Y., Wang, M. & Liao, J. Enantioselective copper-catalyzed methylboration of alkenes. Org. Lett. 20, 1346–1349 (2018).
Green, J. C., Joannou, M. V., Murray, S. A., Joseph, M. Z. & Meek, S. J. Enantio- and diastereoselective synthesis of hydroxy bis(boronates) via Cu-catalyzed tandem borylation/1,2-addition. ACS Catal. 7, 4441–4445 (2017).
Huang, Y., Smith, K. B. & Brown, M. K. Copper-catalyzed borylacylation of activated alkenes with acid chlorides. Angew. Chem. Int. Ed. 56, 13314–13318 (2017).
Lee, J. et al. Mechanism-based enhancement of scope and enantioselectivity for reactions involving a copper-substituted stereogenic carbon centre. Nat. Chem. 10, 99–108 (2018).
Kim, N., Han, J. T., Ryu, D. H. & Yun, J. Copper-catalyzed asymmetric borylallylation of vinyl arenes. Org. Lett. 19, 6144–6147 (2017).
Itoh, T., Kanzaki, Y., Shimizu, Y. & Kanai, M. Copper(I)-catalyzed enantio- and diastereodivergent borylative coupling of styrenes and imines. Angew. Chem. Int. Ed. 57, 8265–8269 (2018).
Li, Z. et al. Nickel-catalyzed regio- and enantioselective borylative coupling of terminal alkenes with alkyl halides enabled by an anionic bisoxazoline ligand. J. Am. Chem. Soc. 145, 13603–13614 (2023).
Wang, H., Liu, C.-F., Martin, R. T., Gutierrez, O. & Koh, M. J. Directing group-free catalytic dicarbofunctionalization of unactivated alkenes. Nat. Chem. 14, 188–195 (2022).
Liu, Z., Li, X., Zeng, T. & Engle, K. M. Directed, palladium (II)-catalyzed enantioselective anti-carboboration of alkenyl carbonyl compounds. ACS Catal. 9, 3260–3265 (2019).
Liu, C.-F. et al. Synthesis of tri- and tetrasubstituted stereocentres by nickel-catalysed enantioselective olefin cross-couplings. Nat. Catal. 5, 934–942 (2022).
Jang, W. J., Song, S. M., Moon, J. H., Lee, J. Y. & Yun, J. Copper-catalyzed enantioselective hydroboration of unactivated 1,1-disubstituted alkenes. J. Am. Chem. Soc. 139, 13660–13663 (2017).
Hong, X. & Shi, S.-L. et al. Copper-catalyzed enantioselective Markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 57, 1376–1380 (2018).
Wen, Y., Deng, C., Xie, J. & Kang, X. Recent synthesis developments of organoboron compounds via metal-free catalytic borylation of alkynes and alkenes. Molecules 24, 101 (2019).
Qi, X. & Diao, T. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 10, 8542–8556 (2020).
Lin, C. & Shen, L. Recent progress in transition metal-catalyzed regioselective functionalization of unactivated alkenes/alkynes assisted by bidentate directing groups. ChemCatChem 11, 961–968 (2019).
Nattmann, L. & Cornella, J. Ni(4‑tBustb)3: a robust 16-electron Ni(0) olefin complex for catalysis. Organometallics 39, 3295–3300 (2020).
Ma, X., Kuang, Z. & Song, Q. Recent advances in the construction of fluorinated organoboron compounds. J. Am. Chem. Soc. Au 2, 261–279 (2022).
Hupe, E., Marek, I. & Knochel, P. Diastereoselective reduction of alkenylboronic esters as a new method for controlling the stereochemistry of up to three adjacent centers in cyclic and acyclic molecules. Org. Lett. 4, 2861–2863 (2002).
Denmark, S. E. & Fu, J. Catalytic enantioselective addition of allylic organometallic reagents to aldehydes and ketones. Chem. Rev. 103, 2763–2794 (2003).
Yus, M., González-Gómez, J. C. & Foubelo, F. Diastereoselective allylation of carbonyl compounds and imines: application to the synthesis of natural products. Chem. Rev. 113, 5595–5698 (2013).
Sardini, S. R. & Brown, M. K. Catalyst controlled regiodivergent arylboration of dienes. J. Am. Chem. Soc. 139, 9823–9826 (2017).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Logan, K. M. & Brown, M. K. Catalytic enantioselective arylboration of alkenylarenes. Angew. Chem. Int. Ed. 129, 869–873 (2017).
Chen, L.-A., Lear, A. R., Gao, P. & Brown, M. K. Nickel-catalyzed arylboration of alkenylarenes: synthesis of boron-substituted quaternary carbons and regiodivergent reactions. Angew. Chem. Int. Ed. 58, 10956–10960 (2019).
Simlandy, A. K., Sardini, S. & Brown, M. K. Construction of congested Csp3–Csp3 bonds by a formal Ni-catalyzed alkylboration. Chem. Sci. 12, 5517–5521 (2021).
Sardini, S. R. & Brown, M. K. Nickel-catalyzed arylboration of cyclopentene. Org. Synth. 97, 355–367 (2020).
Xu, J. et al. Selective oxidation of alkenes to carbonyls under mild conditions. Green Chem. 23, 5549–5555 (2021).
Sonawane, R. P. et al. Enantioselective construction of quaternary stereogenic centers from tertiary boronic esters: methodology and applications. Angew. Chem. Int. Ed. 50, 3760–3763 (2011).
Turnbull, B. W. H. & Evans, P. A. Enantioselective rhodium-catalyzed allylic substitution with a nitrile anion: construction of acyclic quaternary carbon stereogenic centers. J. Am. Chem. Soc. 137, 6156–6159 (2015).
Ishibashi, H. et al. A convenient synthesis of 1,3,4,5-tetrahydro-2H-3-benzazepin-2-ones by acid-catalyzed cylization of N-(2-arylethyl)-N-methyl-2-sulfinylacetamides. Chem. Pharm. Bull. 37, 939–943 (1989).
Coote, S. J., Davies, S. G., Middlemiss, D. & Naylor, A. Enantiospecific synthesis of (+)-(R)-1-phenyl-3-methyl-1,2,4,5-tetrahydrobenz[d]azepine from (+)-(S)-N-methyl-1-phenyl ethanolamine (halostachine) via arene chromium tricarbonyl methodology. Tetrahedron Lett. 30, 3581–3588 (1989).
Goode-Romero, G. et al. New information of dopaminergic agents based on quantum chemistry calculations. Sci. Rep. 10, 21581 (2020).
Wang, D. et al. Asymmetric copper-catalyzed intermolecular aminoarylation of styrenes: efficient access to optical 2,2-diarylethylamines. J. Am. Chem. Soc. 139, 6811–6814 (2017).
Belloni, P. N., Jolidon, S., Klaus, M. & Lapierre, J.-M. New gamma selective retinoids. WO patent 2001/083438 A2 (2001).
González-Rodríguez, J., Soengas, R. G. & Rodríguez-Solla, H. A cooperative zinc/catalytic indium system for the stereoselective sequential synthesis of (E)-1,3-dienes from carbonyl compounds. Org. Chem. Front. 8, 591–598 (2021).
Gilmore, J. L. et al. Synthesis and structure–activity relationships of novel indazolyl glucocorticoid receptor partial agonists. Bioorg. Med. Chem. Lett. 23, 5448–5451 (2013).
Sheppeck, J. E. Modulators of glucocorticoid receptor, AP-1, and/or NF-κΒ activity and use thereof. WO patent 2008/057857 Al (2008).
Liu, C.-F. et al. Olefin functionalization/isomerization enables stereoselective alkene synthesis. Nat. Catal. 4, 674–683 (2021).
Tasker, S. Z., Gutierrez, A. C. & Jamison, T. F. Nickel-catalysed Mizoroki–Heck reaction of aryl sulfonates and chlorides with electronically unbiased terminal olefins: high selectivity for branched products. Angew. Chem. Int. Ed. 53, 1858–1861 (2014).
Liu, C.-F., Luo, X., Wang, H. & Koh, M. J. Catalytic regioselective olefin hydroarylation (alkenylation) by sequential carbonickelation-hydride transfer. J. Am. Chem. Soc. 143, 9498–9506 (2021).
Guo, L. et al. General method for enantioselective three-component carboarylation of alkenes enabled by visible-light dual photoredox/nickel catalysis. J. Am. Chem. Soc. 142, 20390–20399 (2020).
Huang, M. et al. Ni-catalyzed borylation of aryl sulfoxides. Chem. Eur. J. 27, 8149–8158 (2021).
Acknowledgements
This research was supported by the Ministry of Education of Singapore Academic Research Fund Tier 2: A-8000941-00-00 (M.J.K.), the National Key Research & Development Program of China: 2019YFA0905100 and the National Natural Science Foundation of China: 92156025 (J.-A.M.), the National Natural Science Foundation of China: 22325110, 92256303, 22171280 (S.-L.S.) and the National Key Research & Development Program of China: 2021YFF0701700 (J.N.).
Author information
Authors and Affiliations
Contributions
M.J.K., X.L. and C.-F.L. conceived the work. X.L., W.M. and Y.-Q.W. developed the method. M.J.K., J.-A.M., S.-L.S. and J.N. directed the investigations. M.J.K. wrote the manuscript with revisions provided by the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Guoyin Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1 and 2, experimental data, synthesis and characterization data, NMR spectra, HPLC traces and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, X., Mao, W., Liu, CF. et al. Enantioselective synthesis of multifunctional alkylboronates via N-heterocyclic carbene–nickel-catalysed carboboration of alkenes. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00492-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00492-x