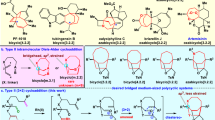
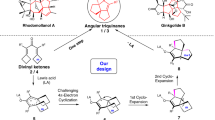
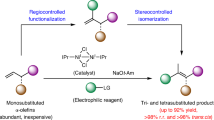
Abstract
Optically active medium-sized cyclic compounds are often found in natural products and are therefore attractive targets in organic synthesis. However, generating these cyclic entities with specific stereochemistry is far from trivial owing to unfavourable entropic factors and competing pathways that favour the formation of rings of lesser size. As a result, conventional ring-forming strategies can be challenging, and alternative methods, such as organocatalytic cycloadditions, have emerged to address these issues. Enantioselective synthesis of medium-sized rings by organocatalytic cycloadditions is a rapidly growing field of research offering opportunities that are complementary to metal-catalysed cycloadditions. Several organocatalytic approaches are available, including enamine/iminium-ion activation, along with catalysis using Lewis and Brønsted acids, hydrogen-bond donors, N-heterocyclic carbenes, and nucleophilic phosphines and amines. Here we discuss the ability of organocatalytic cycloadditions to synthesize stereodefined medium-sized ring architectures, critically evaluate current synthetic strategies, and highlight avenues for further development.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Morrison, K. C. & Hergenrother, P. J. Natural products as starting points for the synthesis of complex and diverse compounds. Nat. Prod. Rep. 31, 6–14 (2014).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020).
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M. & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216 (2021).
Li, J. W.-H. & Vederas, J. C. Drug discovery and natural products: end of an era or an endless frontier? Science 325, 161–165 (2009).
Rosén, J., Gottfries, J., Muresan, S., Backlund, A. & Oprea, T. I. Novel chemical space exploration via natural products. J. Med. Chem. 52, 1953–1962 (2009).
Harvey, A. L. Natural products in drug discovery. Drug Discov. Today 13, 894–901 (2008).
Grigalunas, M., Brakmann, S. & Waldmann, H. Chemical evolution of natural product structure. J. Am. Chem. Soc. 144, 3314–3329 (2022).
Karageorgis, G., Foley, D. J., Laraia, L., Brakmann, S. & Waldmann, H. Pseudo natural products—chemical evolution of natural product structure. Angew. Chem. Int. Ed. 60, 15705–15723 (2021).
Karageorgis, G., Foley, D. J., Laraia, L. & Waldmann, H. Principle and design of pseudo-natural products. Nat. Chem. 12, 227–235 (2020).
Hu, Y.-J., Li, L.-X., Han, J.-C., Min, L. & Li, C.-C. Recent advances in the total synthesis of natural products containing eight-membered carbocycles (2009–2019). Chem. Rev. 120, 5910–5953 (2020).
de Oliveira, K. T., Servilha, B. M., de C. Alves, L., Desiderá, A. L. & Brocksom, T. J. in Studies in Natural Products Chemistry Vol. 42 (ed. Rahman, A.) 421–463 (Elsevier, 2014).
Kaur, N. 8-Membered Heterocycle Synthesis (Elsevier, 2023).
Chen, Y., Rosenkranz, C., Hirte, S. & Kirchmair, J. Ring systems in natural products: structural diversity, physicochemical properties and coverage by synthetic compounds. Nat. Prod. Rep. 39, 1544–1556 (2022).
Yet, L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 100, 2963–3008 (2000).
Engler, E. M., Andose, J. D. & Schleyer, P. V. R. Critical evaluation of molecular mechanics. J. Am. Chem. Soc. 95, 8005–8025 (1973).
Galli, C. & Mandolini, L. The role of ring strain on the ease of ring closure of bifunctional chain molecules. Eur. J. Org. Chem. 2000, 3117–3125 (2000).
Illuminati, G. & Mandolini, L. Ring closure reactions of bifunctional chain molecules. Acc. Chem. Res. 14, 95–102 (1981).
Hendrickson, J. B. Molecular geometry. V. Evaluation of functions and conformations of medium rings. J. Am. Chem. Soc. 89, 7036–7043 (1967).
Winnik, M. A. Cyclization and the conformation of hydrocarbon chains. Chem. Rev. 81, 491–524 (1981).
Wiberg, K. B. The C7−C10 cycloalkanes revisited. J. Org. Chem. 68, 9322–9329 (2003).
Anet, F. A. L. & Krane, J. Strain energy calculation of conformations and conformational changes in cyclooctane. Tetrahedron Lett. 14, 5029–5032 (1973).
Saunders, M. Searching for conformers of nine- to twelve-ring hydrocarbons on the MM2 and MM3 energy surfaces: stochastic search for interconversion pathways. J. Comput. Chem. 12, 645–663 (1991).
Toromanoff, E. Dynamic stereochemistry of the 5-, 6- and 7-membered rings using the torsion angle notation. Tetrahedron 36, 2809–2931 (1980).
Zhang, Z. et al. Construction of bridged polycycles through dearomatization strategies. Org. Biomol. Chem. 19, 3960–3982 (2021).
Liu, J., Liu, X., Wu, J. & Li, C.-C. Total synthesis of natural products containing a bridgehead double bond. Chem 6, 579–615 (2020).
Min, L., Liu, X. & Li, C.-C. Total synthesis of natural products with bridged bicyclo[m.n.1] ring systems via type II [5 + 2] cycloaddition. Acc. Chem. Res. 53, 703–718 (2020).
Presset, M., Coquerel, Y. & Rodriguez, J. Syntheses and applications of functionalized bicyclo[3.2.1]octanes: thirteen years of progress. Chem. Rev. 113, 525–595 (2013).
Ruiz, M., López-Alvarado, P., Giorgi, G. & Menéndez, J. C. Domino reactions for the synthesis of bridged bicyclic frameworks: fast access to bicyclo[n.3.1]alkanes. Chem. Soc. Rev. 40, 3445–3454 (2011).
Narayan, R., Potowski, M., Jia, Z.-J., Antonchick, A. P. & Waldmann, H. Catalytic enantioselective 1,3-dipolar cycloadditions of azomethine ylides for biology-oriented synthesis. Acc. Chem. Res. 47, 1296–1310 (2014).
Held, F. E. & Tsogoeva, S. B. Asymmetric cycloaddition reactions catalyzed by bifunctional thiourea and squaramide organocatalysts: recent advances. Catal. Sci. Technol. 6, 645–667 (2016).
Harmata, M. Fun with (4 + 3)-cycloadditions. Synlett 30, 532–541 (2019).
Min, L., Hu, Y.-J., Fan, J.-H., Zhang, W. & Li, C.-C. Synthetic applications of type II intramolecular cycloadditions. Chem. Soc. Rev. 49, 7015–7043 (2020).
Bejcek, L. P. & Murelli, R. P. Oxidopyrylium [5 + 2] cycloaddition chemistry: historical perspective and recent advances (2008–2018). Tetrahedron 74, 2501–2521 (2018).
Wang, N., Wu, Z., Wang, J., Ullah, N. & Lu, Y. Recent applications of asymmetric organocatalytic annulation reactions in natural product synthesis. Chem. Soc. Rev. 50, 9766–9793 (2021).
Moyano, A. & Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 111, 4703–4832 (2011).
Yao, T., Li, J., Jiang, C. & Zhao, C. Recent advances for the catalytic asymmetric construction of medium-sized rings. J. Catal. 2, 2929–2964 (2022).
Tan, W., Zhang, J.-Y., Gao, C.-H. & Shi, F. Progress in organocatalytic asymmetric (4 + 3) cycloadditions for the enantioselective construction of seven-membered rings. Sci. China Chem. 66, 966–992 (2023).
Wei, Y. & Shi, M. Organocatalytic Cycloadditions for Synthesis of Carbo‐ and Heterocycles (Wiley, 2018).
McNaught, A. D. & Wilkinson, A. Compendium of Chemical Terminology. Vol. 1669 (Blackwell Science, 1997).
Jessen, N. I., McLeod, D. & Jørgensen, K. A. Higher-order cycloadditions in the age of catalysis. Chem 8, 20–30 (2022).
Palazzo, T. A., Mose, R. & Jørgensen, K. A. Cycloaddition reactions: why is it so challenging to move from six to ten electrons? Angew. Chem. Int. Ed. 56, 10033–10038 (2017).
McLeod, D. et al. Expanding the frontiers of higher-order cycloadditions. Acc. Chem. Res. 52, 3488–3501 (2019).
Frankowski, S., Romaniszyn, M., Skrzyńska, A. & Albrecht, Ł. The game of electrons: organocatalytic higher-order cycloadditions involving fulvene- and tropone-derived systems. Chem. Eur. J. 26, 2120–2132 (2020).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Abbasov, M. E. & Romo, D. The ever-expanding role of asymmetric covalent organocatalysis in scalable, natural product synthesis. Nat. Prod. Rep. 31, 1318–1327 (2014).
Reyes-Rodríguez, G. J., Rezayee, N. M., Vidal-Albalat, A. & Jørgensen, K. A. Prevalence of diarylprolinol silyl ethers as catalysts in total synthesis and patents. Chem. Rev. 119, 4221–4260 (2019).
Gaunt, M. J., Johansson, C. C. C., McNally, A. & Vo, N. T. Enantioselective organocatalysis. Drug Discov. Today 12, 8–27 (2007).
Yao, T., Li, J., Jiang, C. & Zhao, C. Recent advances for the catalytic asymmetric construction of medium-sized rings. Chem Catal. 2, 2929–2964 (2022).
Lelais, G. & MacMillan, D. W. C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichim. Acta 39, 79–87 (2006).
List, B. Enamine catalysis is a powerful strategy for the catalytic generation and use of carbanion equivalents. Acc. Chem. Res. 37, 548–557 (2004).
Jensen, K. L., Dickmeiss, G., Jiang, H., Albrecht, Ł. & Jørgensen, K. A. The diarylprolinol silyl ether system: a general organocatalyst. Acc. Chem. Res. 45, 248–264 (2012).
Burns, N. Z., Witten, M. R. & Jacobsen, E. N. Dual catalysis in enantioselective oxidopyrylium-based [5 + 2] cycloadditions. J. Am. Chem. Soc. 133, 14578–14581 (2011).
Witten, M. R. & Jacobsen, E. N. Catalytic asymmetric synthesis of 8-oxabicyclooctanes by intermolecular [5 + 2] pyrylium cycloadditions. Angew. Chem. Int. Ed. 53, 5912–5916 (2014).
McLeod, D. et al. Enantioselective 1,3-dipolar [6 + 4] cycloaddition of pyrylium ions and fulvenes towards cyclooctanoids. Chem. Eur. J. 26, 11417–11422 (2020).
Mose, R. et al. Organocatalytic stereoselective [8 + 2] and [6 + 4] cycloadditions. Nat. Chem. 9, 487–492 (2017).
Yu, P. et al. Organocatalytic [6 + 4] cycloadditions via zwitterionic intermediates: chemo-, regio- and stereoselectivities. J. Am. Chem. Soc. 140, 13726–13735 (2018).
Chen, X. et al. [8 + 2] versus [4 + 2] cycloadditions of cyclohexadienamines to tropone and heptafulvenes—mechanisms and selectivities. J. Am. Chem. Soc. 143, 934–944 (2021).
Donslund, B. S., Johansen, T. K., Poulsen, P. H., Halskov, K. S. & Jørgensen, K. A. The diarylprolinol silyl ethers: ten years after. Angew. Chem. Int. Ed. 54, 13860–13874 (2015).
Orue, A., Uria, U., Reyes, E., Carrillo, L. & Vicario, J. L. Catalytic enantioselective [5 + 2] cycloaddition between oxidopyrylium ylides and enals under dienamine activation. Angew. Chem. Int. Ed. 54, 3043–3046 (2015).
Gao, Y., Song, X., Yan, R.-J., Du, W. & Chen, Y.-C. Asymmetric β,γ′-regioselective [4 + 3] and [4 + 2] annulations of α-vinylenals via cascade iminium ion-dienamine catalysis. Org. Biomol. Chem. 19, 151–155 (2021).
Donslund, B. S. et al. Organocatalytic enantioselective higher-order cycloadditions of in situ generated amino isobenzofulvenes. Angew. Chem. Int. Ed. 57, 1246–1250 (2018).
Donslund, B. S. et al. Catalytic enantioselective [10 + 4] cycloadditions. Angew. Chem. Int. Ed. 57, 13182–13186 (2018).
Bertuzzi, G. et al. Catalytic enantioselective hetero-[6+4] and -[6+2] cycloadditions for the construction of condensed polycyclic pyrroles, imidazoles and pyrazoles. J. Am. Chem. Soc. 141, 3288–3297 (2019).
Woodward, R. B. & Hoffmann, R. The conservation of orbital symmetry. Angew. Chem. Int. Ed. 8, 781–853 (1969).
Corti, V. et al. Organocatalytic enantioselective thermal [4 + 4] cycloadditions. J. Am. Chem. Soc. 145, 1448–1459 (2023).
Harmata, M., Ghosh, S. K., Hong, X., Wacharasindhu, S. & Kirchhoefer, P. Asymmetric organocatalysis of 4 + 3 cycloaddition reactions. J. Am. Chem. Soc. 125, 2058–2059 (2003).
Sun, W.-B., Wang, X., Sun, B.-F., Zou, J.-P. & Lin, G.-Q. Catalytic asymmetric total synthesis of hedyosumins A, B and C. Org. Lett. 18, 1219–1221 (2016).
Wang, J., Chen, S.-G., Sun, B.-F., Lin, G.-Q. & Shang, Y.-J. Collective total synthesis of englerin A and B, orientalol E and F, and oxyphyllol: application of the organocatalytic [4 + 3] cycloaddition reaction. Chem. Eur. J. 19, 2539–2547 (2013).
Wilson, R. M., Jen, W. S. & MacMillan, D. W. C. Enantioselective organocatalytic intramolecular Diels-Alder reactions. The asymmetric synthesis of solanapyrone D. J. Am. Chem. Soc. 127, 11616–11617 (2005).
Corey, E. J., Shibata, T. & Lee, T. W. Asymmetric Diels-Alder reactions catalyzed by a triflic acid activated chiral oxazaborolidine. J. Am. Chem. Soc. 124, 3808–3809 (2002).
Corey, E. J. Enantioselective catalysis based on cationic oxazaborolidines. Angew. Chem. Int. Ed. 48, 2100–2117 (2009).
Mukherjee, S. & Corey, E. Enantioselective synthesis based on catalysis by chiral oxazaborolidinium cations. Aldrichim. Acta 43, 49–59 (2010).
Brimioulle, R. & Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2 + 2] photocycloaddition reactions. Science 342, 840–843 (2013).
Balskus, E. P. & Jacobsen, E. N. Asymmetric catalysis of the transannular Diels-Alder reaction. Science 317, 1736–1740 (2007).
Snyder, S. A. & Corey, E. J. Concise total syntheses of palominol, dolabellatrienone, β-araneosene and isoedunol via an enantioselective Diels-Alder macrobicyclization. J. Am. Chem. Soc. 128, 740–742 (2006).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric binol-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Del Corte, X., Martínez De Marigorta, E., Palacios, F., Vicario, J. & Maestro, A. An overview of the applications of chiral phosphoric acid organocatalysts in enantioselective additions to C–O and C–N bonds. Org. Chem. Front. 9, 6331–6399 (2022).
Jiménez, E. I. An update on chiral phosphoric acid organocatalyzed stereoselective reactions. Org. Biomol. Chem. 21, 3477–3502 (2023).
Rueping, M., Parmar, D. & Sugiono, E. Asymmetric Brønsted Acid Catalysis (Wiley, 2016).
Dorsch, C. & Schneider, C. in Asymmetric Organocatalysis. Ch. 1 (Wiley, 2023).
Mei, G.-J. et al. Brønsted acid-catalyzed stereoselective [4 + 3] cycloadditions of ortho-hydroxybenzyl alcohols with N,N′-cyclic azomethine imines. Chem. Commun. 53, 2768–2771 (2017).
Villar, L. et al. Enantioselective oxidative (4 + 3) cycloadditions between allenamides and furans through bifunctional hydrogen-bonding/ion-pairing interactions. Angew. Chem. Int. Ed. 56, 10535–10538 (2017).
Gelis, C. et al. Highly diastereo- and enantioselective synthesis of cyclohepta[b]indoles by chiral-phosphoric-acid-catalyzed (4 + 3) cycloaddition. Angew. Chem. Int. Ed. 57, 12121–12125 (2018).
Sun, M. et al. Catalytic asymmetric (4 + 3) cyclizations of in situ generated ortho-quinone methides with 2‐indolylmethanols. Angew. Chem. Int. Ed. 131, 8795–8800 (2019).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis (ACDC): a remarkably general approach to enantioselective synthesis. Isr. J. Chem. 52, 630–638 (2012).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition and applications. Angew. Chem. Int. Ed. 52, 518–533 (2013).
Schreyer, L., Properzi, R. & List, B. IDPi catalysis. Angew. Chem. Int. Ed. 58, 12761–12777 (2019).
Ouyang, J., Maji, R., Leutzsch, M., Mitschke, B. & List, B. Design of an organocatalytic asymmetric (4 + 3) cycloaddition of 2-indolylalcohols with dienolsilanes. J. Am. Chem. Soc. 144, 8460–8466 (2022).
Gribble, G. W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles. Vol. 26 (Springer Science & Business Media, 2010).
Huber, T., Wildermuth, R. E. & Magauer, T. 9-membered carbocycles: strategies and tactics for their synthesis. Chem. Eur. J. 24, 12107–12120 (2018).
Bertuzzi, G., McLeod, D., Mohr, L.-M. & Jørgensen, K. A. Organocatalytic enantioselective 1,3-dipolar [6 + 4] cycloadditions of tropone. Chem. Eur. J. 26, 15491–15496 (2020).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Garcia-Mancheno, O. Anion-Binding Catalysis (Wiley, 2022).
Matador, E., Fernández, R., Lassaletta, J. M. & Monge, D. in Asymmetric Organocatalysis. Ch. 4 (Wiley, 2023).
Banik, S. M., Levina, A., Hyde, A. M. & Jacobsen, E. N. Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 358, 761–764 (2017).
Jeyaraman, R. & Avila, S. Chemistry of 3-azabicyclo[3.3.1]nonanes. Chem. Rev. 81, 149–174 (1981).
Tan, J.-P. et al. Asymmetric synthesis of N-bridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 13, 357 (2022).
Qi, S.-S. et al. Catalytic asymmetric conjugate addition/hydroalkoxylation sequence: expeditious access to enantioenriched eight-membered cyclic ether derivatives. Org. Lett. 23, 2471–2476 (2021).
Breslow, R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J. Am. Chem. Soc. 80, 3719–3726 (1958).
Ryan, S. J., Candish, L. & Lupton, D. W. Acyl anion free N-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 42, 4906–4917 (2013).
Enders, D., Niemeier, O. & Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 107, 5606–5655 (2007).
Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 115, 9307–9387 (2015).
Chen, X.-Y., Gao, Z.-H. & Ye, S. Bifunctional N-heterocyclic carbenes derived from l-pyroglutamic acid and their applications in enantioselective organocatalysis. Acc. Chem. Res. 53, 690–702 (2020).
Hong, L., Liu, J.-Y., Hong-Yu, L. & Peng-Fei, X. Recent developments in N-heterocyclic carbene and transition-metal cooperative catalysis. Acta Chim. Sin. 76, 831–837 (2018).
Dhayalan, V., Dandela, R., Sharma, D. & Chatterjee, R. Recent advances in enantioselective organocatalytic reactions enabled by N-heterocyclic carbenes (NHCs) containing triazolium motifs. Synthesis 54, 4129–4166 (2022).
Chen, X., Wang, H., Jin, Z. & Chi, Y. R. N-heterocyclic carbene organocatalysis: activation modes and typical reactive intermediates. Chin. J. Chem. 38, 1167–1202 (2020).
Reyes, E., Uria, U., Carrillo, L. & Vicario, J. L. Enantioselective cascade reactions under N-heterocyclic carbene catalysis. Synthesis 49, 451–471 (2017).
Yao, T., Li, J., Wang, L. & Zhao, C. Recent advances for the construction of seven-membered ring catalyzed by N-heterocyclic carbenes. Chin. J. Org. Chem. 42, 925–944 (2022).
Izquierdo, J., Orue, A. & Scheidt, K. A. A dual Lewis base activation strategy for enantioselective carbene-catalyzed annulations. J. Am. Chem. Soc. 135, 10634–10637 (2013).
Lv, H., Jia, W.-Q., Sun, L.-H. & Ye, S. N-heterocyclic carbene catalyzed [4 + 3] annulation of enals and o-quinone methides: highly enantioselective synthesis of benzo-ε-lactones. Angew. Chem. Int. Ed. 125, 8769–8772 (2013).
Wang, M., Huang, Z., Xu, J. & Chi, Y. R. N-heterocyclic carbene-catalyzed [3 + 4] cycloaddition and kinetic resolution of azomethine imines. J. Am. Chem. Soc. 136, 1214–1217 (2014).
Guo, C., Sahoo, B., Daniliuc, C. G. & Glorius, F. N-heterocyclic carbene catalyzed switchable reactions of enals with azoalkenes: formal [4 + 3] and [4 + 1] annulations for the synthesis of 1,2-diazepines and pyrazoles. J. Am. Chem. Soc. 136, 17402–17405 (2014).
Wang, M., Rong, Z.-Q. & Zhao, Y. Stereoselective synthesis of ε-lactones or spiro-heterocycles through NHC-catalyzed annulation: divergent reactivity by catalyst control. Chem. Commun. 50, 15309–15312 (2014).
Liang, Z.-Q., Gao, Z.-H., Jia, W.-Q. & Ye, S. Bifunctional N-heterocyclic carbene catalyzed [3 + 4] annulation of enals and aurones. Chem. Eur. J. 21, 1868–1872 (2015).
Liang, Z.-Q., Yi, L., Chen, K.-Q. & Ye, S. N-heterocyclic carbene-catalyzed [3 + 4] annulation of enals and alkenyl thiazolones: enantioselective synthesis of thiazole-fused ε-lactones. J. Org. Chem. 81, 4841–4846 (2016).
Wang, L. et al. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3 + 4] annulation reactions. Angew. Chem. Int. Ed. 128, 11276–11280 (2016).
Liu, Q., Li, S., Chen, X.-Y., Rissanen, K. & Enders, D. Asymmetric synthesis of spiro-oxindole-ε-lactones through N-heterocyclic carbene catalysis. Org. Lett. 20, 3622–3626 (2018).
Li, W. et al. NHC-catalyzed enantioselective [4 + 3] cycloaddition of ortho-hydroxyphenyl substituted para-quinone methides with isatin-derived enals. Adv. Synth. Catal. 360, 2460–2464 (2018).
Gao, Z.-H. et al. Enantioselective N-heterocyclic carbene-catalyzed synthesis of spirocyclic oxindole-benzofuroazepinones. J. Org. Chem. 83, 15225–15235 (2018).
Chen, K.-Q., Gao, Z.-H. & Ye, S. Bifunctional N-heterocyclic carbene catalyzed [3 + 4] annulation of enals with azadienes: enantioselective synthesis of benzofuroazepinones. Org. Chem. Front. 6, 405–409 (2019).
Wang, Z., Xu, X. & Kwon, O. Phosphine catalysis of allenes with electrophiles. Chem. Soc. Rev. 43, 2927–2940 (2014).
Guo, H., Fan, Y. C., Sun, Z., Wu, Y. & Kwon, O. Phosphine organocatalysis. Chem. Rev. 118, 10049–10293 (2018).
France, S., Guerin, D. J., Miller, S. J. & Lectka, T. Nucleophilic chiral amines as catalysts in asymmetric synthesis. Chem. Rev. 103, 2985–3012 (2003).
Yan, R.-J., Liu, B.-X., Xiao, B.-X., Du, W. & Chen, Y.-C. Asymmetric (4 + 3) and (4 + 1) annulations of isatin-derived Morita-Baylis-Hillman carbonates to construct diverse chiral heterocyclic frameworks. Org. Lett. 22, 4240–4244 (2020).
Yuan, C. et al. Phosphine-catalyzed enantioselective [4 + 3] annulation of allenoates with C,N-cyclic azomethine imines: synthesis of quinazoline-based tricyclic heterocycles. Org. Lett. 18, 5644–5647 (2016).
Ni, H. et al. Enantioselective phosphine-catalyzed formal [4 + 4] annulation of α,β-unsaturated imines and allene ketones: construction of eight-membered rings. Angew. Chem. Int. Ed. 56, 14222–14226 (2017).
Jia, R.-L., Liu, Q.-L., Yang, L.-W., Deng, S. & Song, Y. [6 + 3] annulations of Morita-Baylis-Hillman carbonates and dicyanoheptafulvene. Org. Biomol. Chem. 19, 9867–9871 (2021).
Acknowledgements
K.A.J. acknowledges a Villum Investigator grant (no. 25867), the Novo Nordisk Foundation, FNU and Aarhus University. J.O. acknowledges support from MUNI/A/1096/2022 (MUNI Brno – Specific research) and the Erasmus+ programme of the European Union. M.E. acknowledges the support of MOV_CA_2021_1_171965 and MIA-322.
Author information
Authors and Affiliations
Contributions
K.A.J. supervised the process. All authors analysed the topic, contributed to the discussions and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jose Vicario and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Otevrel, J., Eugui, M., Ričko, S. et al. Enantioselective organocatalytic cycloadditions for the synthesis of medium-sized rings. Nat. Synth 2, 1142–1158 (2023). https://doi.org/10.1038/s44160-023-00416-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00416-1