Abstract
Background
The objective of this systematic review is to identify prognostic factors among women and their offspring affected by gestational diabetes mellitus (GDM), focusing on endpoints of cardiovascular disease (CVD) and type 2 diabetes (T2D) for women, and cardiometabolic profile for offspring.
Methods
This review included studies published in English language from January 1st, 1990, through September 30th, 2021, that focused on the above outcomes of interest with respect to sociodemographic factors, lifestyle and behavioral characteristics, traditional clinical traits, and ‘omics biomarkers in the mothers and offspring during the perinatal/postpartum periods and across the lifecourse. Studies that did not report associations of prognostic factors with outcomes of interest among GDM-exposed women or children were excluded.
Results
Here, we identified 109 publications comprising 98 observational studies and 11 randomized-controlled trials. Findings indicate that GDM severity, maternal obesity, race/ethnicity, and unhealthy diet and physical activity levels predict T2D and CVD in women, and greater cardiometabolic risk in offspring. However, using the Diabetes Canada 2018 Clinical Practice Guidelines for studies, the level of evidence was low due to potential for confounding, reverse causation, and selection biases.
Conclusions
GDM pregnancies with greater severity, as well as those accompanied by maternal obesity, unhealthy diet, and low physical activity, as well as cases that occur among women who identify as racial/ethnic minorities are associated with worse cardiometabolic prognosis in mothers and offspring. However, given the low quality of evidence, prospective studies with detailed covariate data collection and high fidelity of follow-up are warranted.
Plain language summary
Gestational diabetes mellitus (GDM) occurs when levels of sugar in the blood are high during pregnancy. We sought to identify factors associated with short- and long-term cardiometabolic disease risk, health conditions that involve heart-related issues and complications in bodily function, among women with GDM and their offspring. We reviewed publications on factors related to type 2 diabetes (T2D) and cardiovascular disease (CVD) risk among women with GDM, and additionally assessed body composition in offspring of women with GDM. We found that GDM severity, maternal obesity, self-identified race/ethnicity, poor diet, and low physical activity levels predict postpartum T2D and CVD in the women, and unfavorable long-term cardiometabolic disease risk in offspring. The quality of evidence was poor, emphasizing a need for high-quality research capturing detailed short- and long-term outcome data to facilitate preventative interventions to improve health of women and children.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM), a state of hyperglycemia due to insufficient insulin secretion and/or insulin resistance that occurs during pregnancy, is the most common metabolic disorder of pregnancy, affecting 6–12% of pregnancies globally1,2. A diagnosis of GDM is not only associated with risk of acute pregnancy and delivery complications, but also carries implications for the long-term risk of type 2 diabetes (T2D)3,4 and cardiovascular disease (CVD)5. Additionally, offspring exposed to GDM in utero have higher adiposity and a worse metabolic profile across the life course than their unexposed counterparts6,7. The wide-ranging and intergenerational sequelae of GDM-affected pregnancies emphasize the importance of characterizing not only the short- and long-term consequences of this common pregnancy complication. Further, identification of bellwethers of such consequences will facilitate preventive intervention of such comorbidities and complications, also known as disease prognosis.
Recent technological advancements have improved the capacity to comprehensively assess physiology. In turn, these developments facilitated the ability to harness metabolic heterogeneity – the phenomenon of interest to precision medicine by which similar exposures and risk factors yield differential health sequelae across individuals. In the context of GDM prognosis, this effort requires the identification of prognostic factors and biomarkers among women with a history of GDM and/or their offspring who were exposed to GDM in utero that may serve as both causal and non-causal indicators of future health risks.
Recognizing the relevance of metabolic heterogeneity in accurate and precise assessment of disease prediction, diagnosis, treatment, and prognosis, the Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine8. This Systematic Review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine9.
Thus, in an effort to evaluate prognostic factors to better understand health risks related to postpartum and long-term cardiometabolic health outcomes among mothers with GDM and her offspring, we conducted a systematic review that synthesizes evidence from empirical research papers published through September 1st, 2021, to evaluate and identify prognostic conditions, risk factors, and biomarkers among women and offspring affected by GDM pregnancies, focusing on clinical endpoints of CVD and T2D among women with a history of GDM; and adiposity and cardiometabolic risk profile among offspring exposed to GDM in utero. Overall, we find that GDM severity, maternal obesity, self-identified race/ethnicity, poor diet, and low physical activity levels predict postpartum T2D and CVD in the women, and unfavorable long-term cardiometabolic health in offspring with GDM exposure.
Methods
Systematic review protocol development
We registered our search strategy and systematic review protocol to PROSPERO CRD4202127609410. We developed a systematic review protocol to comprehensively include and evaluate individual research studies reporting on risk factors for long-term clinical outcomes in women with GDM and a range of cardiometabolic health and anthropometric outcomes in GDM-exposed offspring. Nota bene, ADA/EASD PDMI is committed to using inclusive language, especially in relation to gender. We choose to use gendered terminology throughout the article following the rationale for using gendered language in studies of maternal and child health, including but not limited to reducing risk of exposure misclassification and avoidance of dehumanizing terms11. Further, most of the original studies reviewed used ‘women’ as their terminology to describe their population, as GDM per definition occurs in pregnancy which can only occurs in individuals that are female at birth. In this review, we use the term ‘women’ throughout, but acknowledge that not all individuals who experienced a pregnancy may self-identify as a woman.
Our strategy aimed to identify two broad categories of empirical studies: (1) populations of women with a history of prior GDM that investigated additional exposures or risk factors for incident postpartum T2D or CVD; (2) populations comprising offspring exposed to GDM in utero that investigated additional exposures or risk factors for an adverse cardiometabolic profile. Studies including pregnancies unaffected by GDM were eligible only if results were included for GDM subgroups.
Prognostic factors of interest, hereafter also referred to as exposures, included sociodemographic factors, lifestyle and behavioral characteristics, traditional clinical traits, and ‘omics biomarkers. We considered these prognostic factors during the perinatal/postpartum periods and across the lifecourse for both the mothers and offspring. Maternal outcomes of interest were incident T2D or CVD, including study-specific composites of clinical cardiovascular events, non-fatal and fatal myocardial infarction or stroke, and chronic kidney disease (CKD). For offspring, we were interested in outcomes reported 12 weeks of age and older, and limited to anthropometrics, glycemic and cardiometabolic traits or biomarkers, and incident metabolic syndrome (MetS), T2D, or CVD.
Data sources, search strategy, and screening criteria
We developed search terms for Medline EMBASE, and Cochrane CENTRAL (Supplementary Data 1) for eligible citations published in English language from January 1st, 1990, through September 30th, 2021. References of accepted manuscripts and relevant systematic reviews published within the past 2 years were screened to identify additional citations. We included prospective and retrospective observational studies identifying factors with incident outcomes of interest in women or offspring exposed to GDM. We excluded cross-sectional analyses among populations with prevalent disease outcomes or traits. While studies could include non-GDM exposed pregnancies, those without subgroup findings exclusively among GDM pregnancies were excluded. We also included interventions prospectively comparing effects of a treatment assignment on the outcome. Exclusion criteria comprised studies with outcomes <6 weeks postpartum, maternal studies reporting only intermediate phenotypes, glycemic traits, or cardiometabolic biomarkers, and studies in offspring that only assessed endpoints outside of the cardiometabolic outcomes of interest (e.g., neurodevelopment, allergic disease). Using these, two independent reviewers conducted screening at the title abstract level. For accepted citations, two independent reviewers implemented screening of the full manuscripts. Conflicts at all screening stages were resolved by a third reviewer. All screening was conducted in the Covidence online systematic review tracking platform.
Data extraction and synthesis of results
We developed and piloted a data extraction template for eligible manuscripts. Data included manuscript information, study level details and design, population enrollment and characteristics, exposure and outcome ascertainment and diagnosis criteria, follow-up time of outcome assessment since index GDM pregnancy and other pertinent details. We indicated the population in which outcomes were assessed (e.g., maternal, offspring, or both), and recorded the exposures that were investigated in four broad categories: (i) social/genetics factors across the life course; (ii) all factors in perinatal/postpartum window; (iii) long-term maternal exposures; and (iv) long-term offspring exposures.
Quality assessment (risk of bias) and synthesis
We assessed the quality of each study using the Joanna Briggs Institute’s (JBI) critical appraisal tools for cohort studies and randomized controlled trials (RCTs)9. For cohort studies, we assessed quality based on 11 items which evaluated population recruitment, exposure and outcome ascertainment, confounding, statistical methodology, and follow-up. For the RCTs, the JBI criteria evaluated 13 items which assessed selection and allocation, intervention, administration, outcome ascertainment, follow-up, and statistical analysis. Each JBI item was categorized as, ‘Yes,’ ‘No,’ ‘Unclear,’ or ‘Not applicable’ following the guidelines. Any uncertainty in assessment was further discussed by the full research team.
Overall evidence certainty assessment and synthesis
The certainty of evidence was determined using the Diabetes Canada 2018 Clinical Practice Guidelines for studies12. Levels were based on study design and criteria focused on inception cohort of patients presenting GDM but without outcomes of interest, inclusion/exclusion reproducibility, follow-up of at least 80% of participants and assessment of loss to follow-up, adjustment for confounding factors, and reproducible outcome measures. Scoring ranged from level 1 to 4, with Level 1 indicating the highest certainty of evidence and Level 4 indicating the lowest certainty of evidence. Details on the criteria and guidelines are in Supplementary Table 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
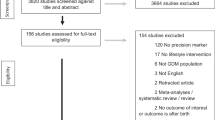
Of the 8141 studies identified, five were excluded due to duplication (Fig. 1). Another 7770 were excluded following title and abstract review. The remaining 366 studies were reviewed in full, of which 106 studies met the inclusion criteria through the database search. An additional three studies were identified through manual search. A total of 109 studies were included in this review.
Of the 109 included, 98 were observational studies and 11 were RCTs (Supplementary Data 2 and 3). Of the studies, 51 focused on maternal outcomes and 38 focused on offspring outcomes. Of the RCTs, three evaluated maternal outcomes and eight assessed offspring outcomes. Studies included data from primarily from white populations from North America and Europe. Sample sizes of the eligible studies ranged from 26 to 23,302.
Maternal outcomes
Maternal type 2 diabetes
Forty-nine observational studies (Supplementary Data 4) and two RCTs (Supplementary Data 5) assessed sociodemographic, lifestyle, clinical, and pregnancy characteristics associated with the risk of T2D among GDM women. The most frequently studied characteristics were maternal BMI and GDM severity. All observational studies that assessed maternal BMI as a prognostic factor showed that higher maternal BMI prior to and/or during pregnancy, and later in the lifecourse predicted higher risk of T2D. One observational study13 further demonstrated that a greater pre-pregnancy weight increased the risk of T2D, though this study did not observe a significant association of gestational weight gain with T2D (Supplementary Data 4). Seventeen observational studies, including one that derived a composite risk score for future T2D risk14, assessed GDM severity in relation to risk of T2D. Findings indicate that more severe GDM, measured by either clinical markers assessing degree of hyperglycemia or need for insulin treatment, predicts risk of developing T2D (Supplementary Data 4). Fewer studies examined the role of lifestyle behaviors and prenatal clinical characteristics. Four observational studies15,16,17,18 investigated the role of self-identified race/ethnicity – which we view as social constructs as opposed to biological forms of determinism – for the risk of T2D, two of which showed no significant associations15,17 and two suggested that the risk was higher among women with non-white European ancestry16,18 (Supplementary Data 4).
Four19,20,21,22 of seven17,19,20,21,22,23,24 observational studies that focused on prognostic value of pregnancy or delivery complications reported that additional pregnancy complications beyond GDM conferred higher risk of T2D. The pregnancy complications assessed varied across reports including stillbirth, gestational hypertension, and cesarian section. Seven studies explored the role of parity24,25,26,27,28,29,30, of which five25,26,27,28,30 found that higher parity predicted risk of T2D. Four observational studies31,32,33,34 showed that breastfeeding was associated with a reduced risk of developing T2D in later life. Two observational studies35,36 and one RCT37 assessed associations of healthy dietary patterns during mid-life with risk of incident T2D among women with a history of GDM but showed inconsistent results. Ten studies assessed biomarkers of T2D risk14,30,38,39,40,41,42,43,44,45, including metabolomics, lipidomics, sICAM and sE-selectin, and proinsulin-to-insulin ratio.
Maternal cardiovascular diseases
Six observational studies19,46,47,48,49,50 explored the role of sociodemographic, lifestyle, and pregnancy characteristics in future risk of CVD among women with GDM (Supplementary Data 6). Two studies identified maternal BMI before46 and during48 pregnancy as risk factors for future CVD, in which women with overweight or obesity, in addition to GDM, have a higher risk of CVD as compared to normal weight women with GDM. One study47 further showed that a healthy lifestyle – i.e., healthy diet, physical activity, and being a non-smoker – was associated with a lower risk of CVD. Two studies showed that pregnancy complications—namely, gestational hypertension50 and stillbirth19—predicted risk of CVD. No effect modification was identified with respect to family history of CVD47 or chronic hypertension48.
Quality of studies conducted and certainty of evidence in women with a history of GDM
The quality of studies for prognostic factors indicative of future T2D or CVD risk is low and the overall certainty of evidence ranked between Levels 3 and 4 according to the Diabetes Canada 2018 Clinical Practice Guidelines12. (Fig. 2 for observational studies; Fig. 3 for RCTs). Most current literature were based on retrospective studies leveraging registry data and observational cohort studies, both of which are vulnerable to bias due to residual confounding, reverse causation bias by pre-existing conditions, and other characteristics around the time of pregnancy and GDM diagnoses.
Offspring outcomes
Anthropometry and body composition
In comparison to the large maternal literature, relatively few studies focused on prognostic factors associated with suboptimal offspring body composition among those exposed to GDM in utero. Forty observational studies (Supplementary Data 7) and five RCTs (Supplementary Data 8) examined associations of sociodemographic, lifestyle, clinical and pregnancy characteristics associated with anthropometric outcomes in offspring of GDM women. The RCTs, by nature, also enabled assessment of the effect of GDM treatment type (e.g., Metformin vs. insulin; dietary advice, glucose monitoring, and insulin therapy vs. routine care) on offspring outcomes.
The most studied associations included maternal BMI, GDM severity, breastfeeding status, and offspring birthweight, in relation to offspring anthropometric outcomes (e.g., BMI and risk of overweight/obesity). Seven observational studies51,52,53,54,55,56,57 found that higher maternal pre-pregnancy BMI was associated with higher adiposity in the offspring, as reflected by a higher BMI, waist circumference or directly-assessed fat mass, and greater risk of overweight or obesity. Nine studies55,56,58,59,60,61,62,63,64 assessed the associations of maternal GDM severity, measured by either clinical markers of hyperglycemia or need for insulin treatment, with offspring body composition, of which four observational studies55,56,63,64 indicated that more severe maternal GDM is associated with a higher offspring BMI and overweight risk. RCTs that evaluated GDM severity and showed no significant association with offspring anthropometry or body composition.
Six55,56,57,60,65,66 of 10 observational studies51,55,56,57,60,65,66,67,68,69 showed that a larger size and/or higher adiposity at birth predicts higher future BMI and risk of overweight among GDM-exposed offspring.
With regards to breastfeeding status, one study69 reported that breastfed offspring with larger size at birth had lower future BMI and lower risk of overweight or obesity. Multiple observational studies showed that exclusive breastfeeding and longer vs. shorter duration of breastfeeding are associated with lower offspring BMI and risk of overweight or obesity (Supplementary Data 7). Additionally, a study in the SWIFT cohort showed that inadequate duration and/or exclusivity of breastfeeding, alone and in combination with consumption of fruit juice or sugar sweetened beverages during the first year of life, predicts higher offspring BMI at ages 2–5 years70. Three studies using data from the Danish National Birth Cohort indicated that maternal prenatal diet consisting of fatty fish71, refined grain72, and sugar-sweetened beverage intake73 were associated with higher offspring BMI, whereas protein intake74 and glycemic index/load75 did not show significant impact on offspring abdominal fat. Finally, one study identified a genetic risk score that predicted higher BMI among offspring exposed to GDM in utero76.
Of the five RCTs testing an effect of GDM treatment on offspring anthropometry and body composition, three77,78,79 yielded null findings and two found that treatment with Metformin, as compared to insulin, was associated with higher offspring adiposity according to skinfold thicknesses80 and weight81 within the first 18 months of life (Supplementary Data 8).
Cardiometabolic profile
We identified fourteen observational studies (Supplementary Data 9) and five RCTs (Supplementary Data 10) that evaluated prognostic risk factors for adverse cardiometabolic outcomes among GDM-exposed offspring. These studies focused on blood pressure, lipids, and glycemic markers in the offspring separately or via a score comprising multiple biomarkers.
Birthweight was the most studied predictor of the offspring prognostic factors, but only two observational studies82,83 showed that a higher birthweight predicted MetS components in offspring later in life. Four observational studies assessed associations of specific maternal dietary components (glycemic index/load75, fish71, magnesium84, and protein74), though no consistent associations were observed in relation to offspring cardiometabolic outcomes. Although one observational study showed that breastfeeding was associated with a lower risk of a MetS phenotype in the offspring85, but this finding was not recapitulated in other observational studies.
Several RCTs compared diet vs. insulin treatment of GDM and showed no significant associations with the development of a MetS phenotype in the offspring (Supplementary Data 10). One RCT86 assessed the effect of a lifestyle intervention comprising exercise and diet counselling for treatment of GDM vs. usual clinical care and found higher risk of unfavorable metabolic outcomes among offspring in the intervention group.
Quality of studies and certainty of evidence conducted in offspring exposed to GDM in utero
We identified low quality of evidence for prognostic factors indicative of future adiposity and cardiometabolic risk among offspring exposed to GDM in utero (Fig. 3 for RCTs; Fig. 4 for observational studies). As with the maternal literature, most studies focusing on offspring outcomes were based on retrospective study designs leveraging registry data and observational cohort studies, both of which can be fraught with residual confounding and reverse causation bias, as well as structural biases like selection and attrition bias. Moreover, the literature of offspring outcomes remains scant and with potentially inadequate durations of follow-up for manifestation of clinically relevant cardiometabolic outcomes, though additional research is warranted. Furthermore, the certainty of evidence for maternal and offspring exposures with cardiometabolic outcomes were scored at Level 412, based on several factors including limited studies, small sample sizes, heterogeneity of study designs, and inadequate statistical methods.
Discussion
Summary
This systematic review sought to identify prognostic risk factors during the perinatal period and across the lifecourse for maternal and offspring cardiovascular and metabolic outcomes among women and offspring affected by GDM pregnancies. We hypothesized that worse glycemic control at the time of GDM diagnosis (i.e., severity of GDM), older maternal age, belonging to a racial/ethnic minority group as proxy of upstream social experiences that trickle down to affect physiology87, unhealthy lifestyle behaviors during the prenatal period (i.e., poor diet quality and low physical activity levels) predict risk of incident type 2 diabetes (T2D) and cardiovascular disease (CVD) among women with a history of GDM, and an unfavorable cardiometabolic profile among offspring exposed to GDM in utero.
The studies identified herein were primarily long-term retrospective and prospective studies. The level of evidence for prognostic risk factors of maternal T2D and CVD and for offspring cardiometabolic risk is low due to unmeasured confounding by lifestyle behaviors, the possibility of reverse causation bias due to pre-existing chronic conditions prior to or at the time of GDM diagnosis. Additionally, for offspring outcomes, the small body of literature on prognostic factors indicative of future adiposity and cardiometabolic risk and major loss to follow-up in both observational and intervention studies.
Maternal outcomes
Among women with GDM, higher BMI at any time in relation to the index pregnancy – i.e., pre-pregnancy, during the index pregnancy including gestational weight gain, and lifecourse measures of weight – predicted higher risk of T2D later in life. GDM severity, typically estimated by use of insulin or higher blood glucose values during the index pregnancy, was consistently associated with higher risk of developing T2D. While few studies assessed race and/or ethnicity as a prognostic risk factor, women of Asian or non-white European descent with a history of GDM had higher risk of future T2D than white women16,18,46. Breastfeeding duration and/or exclusivity was consistently associated with lower risk T2D risk following a GDM diagnosis during pregnancy, though follow-up often ended <2 years postpartum—a period within which occult T2D incidence is relatively low (Supplementary Data 4). Longer duration follow-up is necessary to better evaluate the benefits of breastfeeding on T2D risk. Some observational studies indicated a protective effect of lifestyle factors such as physical activity level during the perinatal and postpartum periods, and compliance with a healthy diet (e.g., adherence to a Mediterranean or DASH-like dietary pattern; the Healthy Eating Index score). However, RCTs investigating the effects of dietary interventions yielded mixed results (Supplementary Data 5). Several observational studies also examined biomarkers of T2D risk following GDM pregnancies, including degree of hyperglycemia at the time of GDM diagnosis, lipids, inflammation, and metabolomics biomarkers38,39,40. However, low certainty of evidence from the studies and lack of replication/validation of findings prevent us from drawing firm conclusions regarding which factors may be the best predictors of future diabetes.
In line with a large literature demonstrating that women with a history of GDM are at higher risk CVD than their non-diabetic counterparts5, studies among women with a history of GDM indicated dose-response associations of maternal BMI – primarily, pre-pregnancy BMI—and GDM severity with these endpoints. However, the extent to which these physiological factors are modifiable remains yet to be determined. Given the paucity of available research on CVD risk in women with a history of GDM, and the low certainty of evidence assessment, this is a research area ripe for investigation.
Quality of maternal studies
We ranked the quality of evidence for prognostic factors indicative of risk of T2D or CVD in women as Level 4 (low)12. Most empirical literature comes predominantly from large health care registries that boast large sample sizes and decades of follow-up. However, they carry high risk of bias in terms of identifying and interpretation specific prognostic characteristics as causal risk factors due to residual confounding due to maternal lifestyle, pre-existing chronic conditions, and other characteristics around time of pregnancy and GDM diagnoses. For example, although maternal hypertension during pregnancy may be a risk factor for T2D or CVD, the association may be explained by maternal BMI, diet quality, physical activity, smoking status, socioeconomic factors, and more. In contrast, there are notable large prospective cohorts, including CARDIA (e.g. refs. 31,46) and the Nurses’ Health Study II (e.g. refs. 47,67), that collected detailed prospective information on the above-mentioned variables, thereby mitigating risk of bias in these studies.
Offspring outcomes
The most common measure of offspring anthropometry was BMI between 2 and 10 years after birth. As with maternal outcomes, observational evidence for offspring indicates that greater GDM severity and higher maternal pre-pregnancy BMI predicts higher offspring adiposity. Yet, interpretation of these findings should be tempered with results of intervention studies showing that GDM treatment did not affect offspring anthropometrics77,78,79. Other frequently studied perinatal predictors of offspring adiposity included birth size and breastfeeding duration/exclusivity. Generally, higher birthweight tended to be associated with higher future BMI55,56,57,65,66. Some observational studies showed a protective effect of breastfeeding against offspring obesity risk during childhood, though this finding was not consistently observed. A few observational studies reported a modifying effect of offspring biological sex on future body composition among children exposed to GDM (e.g.57,88), but the direction of association was not consistent. Of the five RCTs that investigated the effect of GDM treatment on offspring anthropometry and body composition, two found that treatment with Metformin, as compared to insulin, predicted higher offspring adiposity according to skinfold thicknesses80 and weight81 within the first 18 months of life. These results call for additional research to assess long-term offspring outcomes related to pharmaceutical treatments for GDM, especially given findings indicating comparable neonatal outcomes among women treated with Metformin and insulin89.
Most studies that assessed offspring cardiometabolic profile were observational and focused on prognostic factors that occurred during the perinatal/postpartum period, though a few RCTs targeting maternal glycemic control during pregnancy via pharmaceutical treatments and/or lifestyle alterations. Among observational studies (Supplementary Data 9), common prognostic factors included maternal BMI and diet, for which both prognostic factors yielded inconsistent associations with offspring cardiometabolic profile. As with the studies assessing offspring anthropometry and body composition as outcomes, RCTs to prevent GDM among high-risk women generally found minimal effects of the pharmaceutical and/or lifestyle interventions on offspring cardiometabolic profile (Supplementary Data 10). This, again, suggests that additional research is needed to better understand the pathophysiology of maternal GDM, to characterize relevant in utero programming pathways90,91,92, and identify accurate and valid prognostic biomarkers—including those in cord blood—as well as outcomes in offspring that are more relevant to future disease risk6 such as directly-assessed neonatal adiposity92.
Quality of offspring studies
As with the maternal studies, we categorized the literature on prognostic factors for offspring outcomes as being of low quality (Level 4)12. The inconsistent observational findings in conjunction with null results of RCTs targeting prevention of GDM among high-risk women indicate the existence of residual confounding for observational studies, and in the cases of the trials, the possibility that the interventions were developed with a suboptimal endpoint (e.g., a focus on preventing macrosomia based on birth size rather than directly assessed neonatal adiposity). Future work is needed to gain a better understanding of in utero programming mechanisms that may link maternal GDM to offspring adiposity, as well as interventions specifically formulated to prevent neonatal adiposity assessed via gold standard methods such as computed tomography or dual X-ray absorptiometry93,94.
Strengths and limitations of studies included in the systematic review
A key strength of many studies included in this systematic review is the prospective study design, which enhances temporal and causal inference regarding prognostic capacity of the maternal and offspring characteristics and behaviors assessed in studies herein. Additional strengths of some, but not all studies, include multi-ethnic study populations, which enhance generalizability of findings; large sample sizes, which improves capacity to detect biologically relevant associations; and use of gold standard assessments of the maternal and offspring outcomes of interest.
Limitations include the low-grade quality of studies included in this review (residual confounding, reverse causation bias, attrition and selection bias, inadequate duration of follow-up). Additionally, most studies were not designed to explore the long-term prognosis of GDM. Accordingly, many studies comprise post hoc analyses that were likely underpowered to detect smaller but biologically relevant effects of prognostic risk factors solely among mothers and/or offspring exposed to GDM. When screening studies, we also noted that a general limitation of the literature on GDM prognostics in relation to offspring outcomes is assessment of the prognostic variable(s) of interest contemporaneously with outcome assessment, which limits our ability to make causal inference on the effect of the prognostic variable on outcomes of interest. These shortcomings resulted in high risk of bias and low quality of studies.
Strengths and limitations of systematic review approach and methodology
Strengths of the methodology for this systematic review include implementation of at least two independent reviews across all phases of the extraction and assessment process, with an additional review by a third independent reviewer to resolve conflicts; and adherence to well-established assessments of research quality and assessments of bias. Limitations include the exclusively qualitative synthesis of results—a necessity given the relatively small number of studies identified; and as with all systematic reviews, the potential for our conclusions to be impacted by publication bias.
Future directions
Given the low quality of evidence identified in this systematic review, there is need for prospective cohort studies in diverse populations with granular data collection on prognostic risk factors as well as clinical and subclinical outcomes. Additionally, high fidelity of follow-up across the lifecourse, particularly during sensitive windows of development during which there is greater developmental plasticity to respond to external cues95, will shed light on avenues for primordial and/or primary prevention. Finally, consideration of appropriate adjustment covariates depending on the specific prognostic risk factor of interest (e.g., there is discourse regarding whether maternal pre-pregnancy BMI should be included as a covariate in models where GDM severity is the prognostic factor of interest given that these variables share overlapping in utero programming pathways6,91); and appropriate causal inference and analytical approaches to address structural biases that afflict observational study designs95,96.
As interest in the application of precision prognostics to improve health for women and offspring affected by GDM pregnancies grows, there remains a crucial need to establish foundational knowledge regarding traditional prognostic factors which, in turn, will enhance our ability to identify new prognostic biomarkers that improve risk stratification for unfavorable health outcomes among both women and children affected by GDM.
Data availability
The data that support the findings of this study are derived from published, peer-reviewed manuscripts. The search terms used to retrieve studies are found in the Supplementary Data 1 and the list of included studies is described in Supplementary Data 2 and 3. The source data underlying Figs. 2–4 is provided in Supplementary Data 4 to 10. All other relevant data are available from the authors upon request.
References
Zhu, Y. & Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr. Diabetes Rep. 16, 7 (2016).
Wang, H. et al. IDF Diabetes Atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s criteria. Diabetes Res. Clin. Pract. 183, 109050 (2022).
Zhang, C. et al. Diabetes & Women’s Health (DWH) Study: an observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open 9, e025517 (2019).
Vince, K., Poljičanin, T., Brkić, M., Rodin, U. & Matijević, R. Prevalence of diabetes five years after having gestational diabetes during pregnancy—Croatian national study. Prim. Care Diabetes 12, 325–330 (2018).
Kramer, C. K., Campbell, S. & Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 62, 905–914 (2019).
Perng, W., Oken, E. & Dabelea, D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 62, 1779–1788 (2019).
Leybovitz-Haleluya, N., Wainstock, T., Landau, D. & Sheiner, E. Maternal gestational diabetes mellitus and the risk of subsequent pediatric cardiovascular diseases of the offspring: a population-based cohort study with up to 18 years of follow up. Acta Diabetolog. 55, 1037–1042 (2018).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45, 261–266 (2022).
Tobias, D. K. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 29, 2438–2457 (2023).
Tobias D. K., et al. Predictors and risk factors of short-term and long-term outcomes in women with GDM and their offspring. PROSPERO: International prospective register of systematic reviews [ONLINE]. 2021; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021276094. Accessed 12 April 2023.
Gribble, K. D. et al. Effective communication about pregnancy, birth, lactation, breastfeeding and newborn care: the importance of sexed language. Front. Glob. Women’s Health 3, 818856 (2022).
Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 42, S1–S325 (2018).
Eades, C. E., Styles, M., Leese, G. P., Cheyne, H. & Evans, J. M. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: an observational follow-up study. BMC Preg. Childbirth 15, 11 (2015).
Schaefer-Graf, U. M. et al. How do we reduce the number of cases of missed postpartum diabetes in women with recent gestational diabetes mellitus? Diabetes Care 32, 1960–1964 (2009).
Wang, Y. et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Women’s Health (2002) 21, 628–633 (2012).
Lee, A. J., Hiscock, R. J., Wein, P., Walker, S. P. & Permezel, M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes care 30, 878–883 (2007).
Lauenborg, J. et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care 27, 1194–1199 (2004).
Wahlberg, J. et al. Gestational diabetes: glycaemic predictors for fetal macrosomia and maternal risk of future diabetes. Diabetes Res. Clin. Pract. 114, 99–105 (2016).
Pintaudi, B. et al. The long-term effects of stillbirth on women with and without gestational diabetes: a population-based cohort study. Diabetologia 58, 67–74 (2015).
Yuan, X. et al. Gestational hypertension and chronic hypertension on the risk of diabetes among gestational diabetes women. J. Diabetes Complic. 30, 1269–1274 (2016).
Li, W. et al. Nomograms for incident risk of post-partum type 2 diabetes in Chinese women with prior gestational diabetes mellitus. Clin. Endocrinol. 90, 417–424 (2019).
Liu, H. et al. Fasting and 2-hour plasma glucose, and HbA1c in pregnancy and the postpartum risk of diabetes among Chinese women with gestational diabetes. Diabetes Res. Clin. Pract. 112, 30–36 (2016).
Lin, P. C., Hung, C. H., Huang, R. D. & Chan, T. F. Predictors of type 2 diabetes among Taiwanese women with prior gestational diabetes mellitus. Jpn J. Nurs Sci. 13, 3–9 (2016).
Pallardo, F. et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 22, 1053–1058 (1999).
Varner, M. W. et al. Pregnancies after the diagnosis of mild gestational diabetes mellitus and risk of cardiometabolic disorders. Obstetr. Gynecol. 129, 273–280 (2017).
Steinhart, J. R., Sugarman, J. R. & Connell, F. A. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care 20, 943–947 (1997).
Chodick, G. et al. The risk of overt diabetes mellitus among women with gestational diabetes: a population-based study. Diabet. Med. 27, 779–785 (2010).
Kawasaki, M. et al. Risk factors during the early postpartum period for type 2 diabetes mellitus in women with gestational diabetes. Endocr. J. 67, 427–437 (2020).
Linné, Y., Barkeling, B. & Rössner, S. Natural course of gestational diabetes mellitus: long term follow up of women in the SPAWN study. BJOG 109, 1227–1231 (2002).
Löbner, K. et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes 55, 792–797 (2006).
Gunderson, E. P. et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Intern. Med. 178, 328–337 (2018).
Ley, S. H. et al. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care 43, 793–798 (2020).
Gunderson, E. P. et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann. Intern. Med. 163, 889–898 (2015).
Shen, Y. et al. Lactation intensity and duration to postpartum diabetes and prediabetes risk in women with gestational diabetes. Diabetes/Metab. Res. Rev. 35, e3115 (2019).
Tobias, D. K. et al. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch. Intern Med. 172, 1566–1572 (2012).
Bao, W. et al. Low carbohydrate-diet scores and long-term risk of type 2 diabetes among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetes Care 39, 43–49 (2016).
Ratner, R. E. et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 93, 4774–4779 (2008).
Tobias, D. K. et al. Dietary intakes and circulating concentrations of branched-chain amino acids in relation to incident type 2 diabetes risk among high-risk women with a history of gestational diabetes mellitus. Clin. Chem. 64, 1203–1210 (2018).
Lai, M. et al. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: a metabolic profiling study. PLoS Med. 17, e1003112 (2020).
Göbl, C. S. et al. Biomarkers of endothelial dysfunction in relation to impaired carbohydrate metabolism following pregnancy with gestational diabetes mellitus. Cardiovasc. Diabetol. 13, 138 (2014).
Hanson, U., Persson, B., Hartling, S. G. & Binder, C. Increased molar proinsulin-to-insulin ratio in women with previous gestational diabetes does not predict later impairment of glucose tolerance. Diabetes Care 19, 17–20 (1996).
Lai, M. et al. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. eLife 9, e59153 (2020).
Allalou, A. et al. A predictive metabolic signature for the transition from gestational diabetes mellitus to type 2 diabetes. Diabetes 65, 2529–2539 (2016).
Lappas, M. et al. The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia 58, 1436–1442 (2015).
Weerasiri, T., Riley, S. F., Sheedy, M. T., Walstab, J. E. & Wein, P. Amniotic fluid insulin values in women with gestational diabetes as a predictor of emerging diabetes mellitus. Aust. N.Z. J. Obstetr. Gynaecol. 33, 358–361 (1993).
Dehmer, E. W. et al. Association between gestational diabetes and incident maternal CKD: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Kidney Dis. 71, 112–122 (2018).
Tobias, D. K. et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern. Med. 177, 1735–1742 (2017).
Fadl, H. et al. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG 121, 1530–1536 (2014).
Kaul, P. et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet. Med. 32, 164–173 (2015).
Pace, R., Brazeau, A. S., Meltzer, S., Rahme, E. & Dasgupta, K. Conjoint associations of gestational diabetes and hypertension with diabetes, hypertension, and cardiovascular disease in parents: a retrospective cohort study. Am. J. Epidemiol. 186, 1115–1124 (2017).
Boerschmann, H., Pflüger, M., Henneberger, L., Ziegler, A. G. & Hummel, S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 33, 1845–1849 (2010).
Kubo, A. et al. Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 37, 2996–3002 (2014).
Leng, J. et al. GDM women’s pre-pregnancy overweight/obesity and gestational weight gain on offspring overweight status. PloS One 10, e0129536 (2015).
Pirkola, J. et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 33, 1115–1121 (2010).
Vohr, B. R. & McGarvey, S. T. Growth patterns of large-for-gestational-age and appropriate-for-gestational-age infants of gestational diabetic mothers and control mothers at age 1 year. Diabetes Care 20, 1066–1072 (1997).
Vohr, B. R., McGarvey, S. T. & Tucker, R. Effects of maternal gestational diabetes on offspring adiposity at 4-7 years of age. Diabetes Care 22, 1284–1291 (1999).
Zhao, Y. L. et al. Maternal gestational diabetes mellitus and overweight and obesity in offspring: a study in Chinese children. J. Dev Origins Health Dis. 6, 479–484 (2015).
Egeland, G. M. & Meltzer, S. J. Following in mother’s footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet. Med. 27, 257–265 (2010).
Landi, S. N. et al. Association of long-term child growth and developmental outcomes with metformin vs insulin treatment for gestational diabetes. JAMA Pediatr. 173, 160–168 (2019).
Schaefer-Graf, U. M. et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 28, 1745–1750 (2005).
Shi, X. et al. Maternal gestational diabetes mellitus and offspring body mass index from 1 to 4 years. Endocr. Pract. 26, 619–626 (2020).
Wroblewska-Seniuk, K., Wender-Ozegowska, E. & Szczapa, J. Long-term effects of diabetes during pregnancy on the offspring. Pediatr. Diabetes 10, 432–440 (2009).
Zhang, S. et al. Maternal glucose during pregnancy and after delivery in women with gestational diabetes mellitus on overweight status of their children. BioMed. Res. Int. 2015, 543038 (2015).
Zhu, Y. et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J. Clin. Nutr. 103, 794–800 (2016).
Chen, Y. L. et al. Adverse pregnancy outcomes on the risk of overweight offspring: a population-based retrospective study in Xiamen, China. Sci. Rep. 10, 1549 (2020).
Hammoud, N. M., de Valk, H. W., Biesma, D. H. & Visser, G. H. Intrauterine adiposity and BMI in 4- to 5-year-old offspring from diabetic pregnancies. Neonatology. 111, 177–181 (2017).
Gillman, M. W., Rifas-Shiman, S., Berkey, C. S., Field, A. E. & Colditz, G. A. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111, e221–e226 (2003).
Hammoud, N. M. et al. Long-term BMI and growth profiles in offspring of women with gestational diabetes. Diabetologia 61, 1037–1045 (2018).
Kaul, P. et al. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia 62, 249–258 (2019).
Vandyousefi, S., Davis, J. N. & Gunderson, E. P. Association of infant diet with subsequent obesity at 2-5 years among children exposed to gestational diabetes: the SWIFT study. Diabetologia 64, 1121–1132 (2021).
Maslova, E. et al. Fish intake in pregnancy and offspring metabolic parameters at age 9-16-does gestational diabetes modify the risk? Nutrients 10, 1534 (2018).
Zhu, Y. et al. Maternal dietary intakes of refined grains during pregnancy and growth through the first 7 y of life among children born to women with gestational diabetes. Am. J. Clin. Nutr. 106, 96–104 (2017).
Zhu, Y. et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int. J. Epidemiol. 46, 1499–1508 (2017).
Maslova, E. et al. Maternal protein intake in pregnancy and offspring metabolic health at age 9-16 y: results from a Danish cohort of gestational diabetes mellitus pregnancies and controls. Am. J. Clin. Nutr. 106, 623–636 (2017).
Maslova, E. et al. Maternal glycemic index and glycemic load in pregnancy and offspring metabolic health in childhood and adolescence—a cohort study of 68,471 mother-offspring dyads from the Danish National Birth Cohort. Eur. J. Clin. Nutr. 73, 1049–1062 (2019).
Liang, Z. et al. Maternal gestational diabetes mellitus modifies the relationship between genetically determined body mass index during pregnancy and childhood obesity. Mayo Clin. Proc. 95, 1877–1887 (2020).
Gillman, M. W. et al. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 33, 964–968 (2010).
Landon, M. B. et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care 38, 445–452 (2015).
Malcolm, J. C., Lawson, M. L., Gaboury, I., Lough, G. & Keely, E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet. Med. 23, 565–570 (2006).
Rowan, J. A. et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care 34, 2279–2284 (2011).
Ijäs, H., Vääräsmäki, M., Saarela, T., Keravuo, R. & Raudaskoski, T. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG 122, 994–1000 (2015).
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–e296 (2005).
Wilk, M. et al. Assessment of selected carbohydrate parameters in children exposed to gestational diabetes in utero. Neuro Endocrinol. Lett. 36, 504–510 (2015).
Del Gobbo, L. C., Song, Y., Elin, R. J., Meltzer, S. J. & Egeland, G. M. Gestational glucose intolerance modifies the association between magnesium and glycemic variables in mothers and daughters 15 years post-partum. Magnes. Res. 25, 54–63 (2012).
Vandyousefi, S. et al. Association of breastfeeding and gestational diabetes mellitus with the prevalence of prediabetes and the metabolic syndrome in offspring of Hispanic mothers. Pediatr. Obes. 14, e12515 (2019).
Grotenfelt, N. E. et al. Effect of maternal lifestyle intervention on metabolic health and adiposity of offspring: findings from the Finnish Gestational Diabetes Prevention Study (RADIEL). Diabetes Metab. 46, 46–53 (2020).
Flanagin, A., Frey, T. & Christiansen, S. L., Committee AMoS. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326, 621–627 (2021).
Nouhjah, S., Shahbazian, H., Latifi, S. M., Malamiri, R. A. & Ghodrati, N. Body mass index growth trajectories from birth through 24 months in Iranian infants of mothers with gestational diabetes mellitus. Diabetes Metab. Syndrome 13, 408–412 (2019).
Gui, J., Liu, Q. & Feng, L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PloS One 8, e64585 (2013).
Francis, E. C., Kechris, K., Jansson, T., Dabelea, D. & Perng, W. Novel metabolic subtypes in pregnant women and risk of early childhood obesity in offspring. JAMA Netw. Open 6, e237030 (2023).
Francis, E. C. et al. Metabolomic profiles in childhood and adolescence are associated with fetal overnutrition. Metabolites 12, 265 (2022).
Erickson, M. L. et al. Maternal metabolic health drives mesenchymal stem cell metabolism and infant fat mass at birth. JCI Insight 6, e146606 (2021).
Colley, D. et al. Assessing body fatness in obese adolescents: alternative methods to dual-energy X-ray absorptiometry. Digest 50, 1–7 (2015).
Willett W. Anthropometric measures and body composition. In: Nutritional Epidemiology. 244–272 (New York: Oxford University Press, 1998).
Laubach, Z. M., Holekamp, K. E., Aris, I. M., Slopen, N. & Perng, W. Applications of conceptual models from lifecourse epidemiology in ecology and evolutionary biology. Biology Lett. 18, 20220194 (2022).
Laubach, Z. M., Murray, E. J., Hoke, K. L., Safran, R. J. & Perng, W. A biologist’s guide to model selection and causal inference. Proc. Biol. Sci. 288, 20202815 (2021).
Acknowledgements
The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL). We also thank Marie-France Hivert for her valuable insights and feedback on this work. In addition, the following individuals were funded by the following sources:
• Zhila Semnani-Azad is funded by Canadian Institutes of Health Research (CIHR) Fellowship.
• Romy Gaillard is funded by the Dutch Diabetes Foundation (grant number 2017.81.002), the Netherlands Organization for Health Research and Development (NWO, ZonMw, grant number 543003109; NWO, ZonMw VIDI 09150172110034) and from the European Union’s Horizon 2020 research and innovation programme under the ERA-NET Cofund action (no 727565), EndObesity, ZonMW the Netherlands (no. 529051026).
• Alice Hughes is funded by a Wellcome Trust GW4-Clinical Academic PhD Fellowship [WT203918].
• Kristen Boyle is funded by R01DK117168.
• Deirdre K. Tobias is funded by ADA-1-19-JDF-115.
• Wei Perng is funded by American Diabetes Association (ADA)−7-22-ICTSPM-08 and National Institutes of Health (NIH) U01 DK134981.
Funders did not play any role in the design of this study or the interpretation of the findings.
Author information
Authors and Affiliations
Consortia
Contributions
Z.S.A., R.G., A.E.H., K.E.B., D.K.T. and W.P. completed the review, extraction, and quality assessment of papers. Z.S.A., R.G. and W.P. drafted the initial version of the manuscript. A.E.H., K.E.B. and D.K.T. provided critical intellectual feedback. All authors approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Delphine Mitanchez, Erica Gunderson, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Semnani-Azad, Z., Gaillard, R., Hughes, A.E. et al. Precision stratification of prognostic risk factors associated with outcomes in gestational diabetes mellitus: a systematic review. Commun Med 4, 9 (2024). https://doi.org/10.1038/s43856-023-00427-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00427-1