Abstract
Background:
Data on seroconversion rates after SARS-CoV-2 infection in young children (<6 years) is scarce. The present study compares seroconversion rates between young children and adults and identifies associated factors.
Methods:
The COALA study (“Corona-outbreak-related examinations in daycare centers”) investigated transmission dynamics of SARS-CoV-2 in daycare centers and associated households (10/2020-06/2021). 114 individuals tested positive for SARS-CoV-2 through PCR either prior to the study period by health authorities or in PCR testing during the study period. Two capillary blood samples were obtained within five weeks consecutively and tested for SARS-CoV-2 IgG-antibodies (second sampling depending on positive PCR). Results from 91 participants (38 young children 1–6 years, 53 adults) were included in the analyses.
Results:
Seroconversion rate in young children is significantly higher than in adults (97.4% versus 66%). High viral load and longer time interval between the probable date of infection and antibody testing are associated with seroconversion.
Conclusions:
Our findings depict substantial development of specific antibodies in young children after SARS-CoV-2 infection. This may provide temporary protection from re-infection for young children or severe disease for this age group.
Plain language summary
When fighting an infectious disease, the immune system often produces antibodies. These proteins circulate in the blood, where they help to clear the infection and generally remain present for several months after recovery. Little is known about how often children younger than 6 years develop antibodies after SARS-CoV-2 infection. The aim of our study was to compare antibody development of young children and adults. We examined blood samples from young children and adults after SARS-CoV-2 outbreaks in daycare centers during the early pandemic (10/2020–06/2021) in Germany. Young children and adults who tested positive for SARS-CoV-2 had two blood samples taken at an interval of five weeks. We found that young children are more likely to develop antibodies after SARS-CoV-2 infection than adults. These findings indicate that young children may be—at least temporarily—protected from re-infection or from a severe course of the disease.
Similar content being viewed by others
Introduction
Seroconversion rate describes the proportion of individuals developing antibodies after antigen contact via an infection or vaccination in a given time period. Determining seroconversion rates after natural infection is important to assess immune protection and to develop and adjust vaccination strategies. Seroconversion after SARS-CoV-2 infection in adults has been described to occur as early as 7 days after symptom onset with seroconversion rates from 50% to 58.8%1,2 and up to 100% 14 days after symptom onset2,3,4. Studies concerning children, however, show a greater variety in their results regarding seroconversion duration and rates. For children and teenagers (0–18 years), seroconversion rates from 37% to 100% have been described, based on blood samples taken 14 days (and up to 172 days) after positive PCR testing or symptom onset5,6,7,8,9. This heterogeneity highlights the need for further investigation. For example, children under 6 years of age present an understudied, albeit relevant population group. Children under the age of 6 years are often enrolled in daycare programs, where SARS-CoV-2 transmission, due to close contact among young children, cannot be fully prevented. Vaccines for children were not approved in the European Union, and thus also in Germany, until November 2021 (5–11-year-olds) and October 2022 (6 months to 4-year-olds). At the same time, in both cases, the STIKO (Standing Committee on Vaccination) did not issue a general vaccination recommendation for the respective age groups10,11.
Generally, data regarding seroconversion after SARS-CoV-2 infection in young children is scarce. The few studies that exist describe seroconversion rates as low as 37.5% one to two months after positive SARS-CoV-2 PCR testing12. Most of the studies focusing on this age group assess children in clinical settings8,12. Only little data is available for young children in non-clinical community settings9,13. Seroconversion rates might also vary between different viral variants14, which requires a continuous updating of seroconversion rates throughout the COVID-19 pandemic.
In addition to age, other potential determinants of seroconversion have been identified, such as sex, time interval between symptom onset or a positive PCR testing and antibody testing, severity of disease, and viral load3,4,5,6,7,8,9,12.
In order to better understand seroconversion in young children, we addressed the following research questions: (a) To what extent do seroconversion rates differ between young children and adults? (b) What factors are associated with seroconversion in young children and adults?
Our results show a significantly higher seroconversion rate in young children than in adults. The analyses depict that seroconversion is associated with viral load (Ct value), time interval between the probable date of infection and antibody testing at follow-up and older age. These results may indicate temporary protection of young children from re-infection or severe disease in the event of re-infection.
Methods
Data used for this analysis were derived from the COALA study and collected between October 2020 and June 2021, when the SARS-CoV-2 ancestral and alpha-variant were predominant in Germany.
Study design
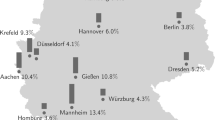
Methods of the underlying COALA study are described elsewhere15. In brief, the COALA study (“Corona-outbreak-related examinations in daycare centers”) was a prospective, longitudinal study, investigating the transmission dynamics of SARS-CoV-2 in 30 child daycare centers and connected households. Therefore, the selection of participants was related to SARS-CoV-2 outbreaks in daycare centers all over Germany. Infection trajectories of affected children and their contacts were monitored via repeated PCR testing, symptom recording, and capillary blood sampling as well as via a written questionnaire about previous SARS-CoV-2 infections and exposure to SARS-CoV-2-infected individuals. The Ethics Committee of the Berlin Medical Association has reviewed the COALA study and approved the implementation of the study (Eth-39/20). Participation in the study was voluntary and all participants were informed about the objectives and contents of the study as well as data protection and gave their written informed consent. Every participant was assigned to a sequential study number (ANR) in order to ensure pseudonymization of the study documents and material. COALA is registered in the German Clinical Trial Register (DRKS-ID = DRKS00023501).
Sample characteristics
In the COALA study, 114 individuals tested positive for SARS-CoV-2 via PCR (45 young children 1-6 years, 69 adults). Antibody results were available for 97 participants (38 young children, 59 adults). For 17 participants (seven young children, ten adults) no measurement of antibodies was performed because no appointment for the blood sampling could be made, blood sampling was refused, or because the sample quality of the blood specimen was insufficient. Participants originated from a non-clinical community setting and were asymptomatic or had mild symptoms of COVID-19 (i.e., rhinitis, headache, sore throat, cough, melalgia, and fever, see also ref. 16).
Sampling
To examine SARS-CoV-2 antibodies, capillary blood was taken at two points of time: The first was taken at the beginning of the study period at the first home visit (=baseline sampling), 4 to 6 days after the testing date of the index case. The index case was defined as the first positive case within the daycare group that was diagnosed with SARS-CoV-2 via PCR and reported to the local health authority. The second blood sample (=follow-up sampling) was taken approximately five weeks after the baseline sampling visit according to the study protocol if the participating individual tested positive for SARS-CoV-2 through PCR either prior to the study period by health authorities or in PCR testing during the study period. (see also Supplementary Fig. 1).
Laboratory analyses
Dried blood samples (DBS) were analyzed for SARS-CoV-2 IgG antibodies using the commercial qualitative antibody assay “Anti-SARS-CoV-2 ELISA (IgG)” (sensitivity 94.4%—blood sample collected >10 days after symptom onset; specificity 99.6%) by the manufacturer Euroimmun (Euroimmun Medizinische Labordiagnostik AG, Lübeck, Germany). The test detects antibodies directed at the spike antigen (S1 antigen). A validation study was performed to verify the applicability of the test for dried blood samples. After evaluation of the results, the ratio threshold for seropositivity provided by the manufacturer (≥1.1) was adapted to ≥0.9417. Indeterminate results were considered negative. The test results are semi-quantitative, i.e., they can only be categorized as positive (≥0.94 ratio) or negative (<0.94 ratio) without quantification in terms of titer determination.
The test quality of the PCR measurements was ensured by measuring human c-myc18.
Determination of seroconversion rates
To establish comparability between the seroconversion rates in young children and adults, we determined the probable date of infection for each participant, considering information on exposure and symptom onset (if any), antibody status at first blood draw, and date of first positive PCR test (for further information see ref. 19). Due to our study design, the timing of the first positive PCR within the course of the infection varied among study participants. As described in other publications, symptoms and symptom onset are no reliable parameters for the onset of SARS-CoV-2 infections in children20,21. Therefore, we decided to determine the individual probable dates of infection as the starting points for analyses of individual infection courses.
To assess a potential association between seroconversion and viral load, we selected the PCR results with the highest viral load (i.e., lowest Ct value; further details see ref. 22) of each participant for the analyses.
Statistics and reproducibility
Characteristics of participants are shown as mean/ median, ±SD (standard deviation) for continuous variables, and as % for categorical variables.
A backward stepwise logistic regression approach was used to identify possible predictors of the outcome seroconversion at testing at follow up. The following variables were taken into consideration: sex, a variable indicating whether the person tested was an adult or a child, viral load (lowest Ct value), presence of symptoms, and the time interval between the probable date of infection and antibody testing at follow-up.
Regardless of the criterion used to determine which variables to exclude, viral load (lowest Ct value), the time interval between the probable date of infection and antibody testing at follow-up, and a variable indicating the person tested being an adult were selected for further analysis, while sex and symptoms were dismissed.
The final model presented in the text was re-run with Firth bias correction23. This correction was used to account for the small sample size in some categories (only one seronegative case in the group of young children). This small sample size also is the reason for presenting results as odds ratios, based on a logistic regression model, and not as relative risks, since the odds ratio estimate is less subject to small sample bias than the risk ratio estimate.
In addition, the multivariable logistic regression analysis was corrected for the unequal distribution of antibody-positive individuals in young children and adults. For this purpose, the indicator variable indicating whether the tested person is an adult was inserted.
Analyses were performed using STATA/SE 17 (StataCorp LLC, College Station TX, USA), as well as R version 4.1.2 (2021-11-01) “Bird Hippie” Copyright (C) 2021 for stepwise backward logistic regression package ‘MASS’ version 7.3-58.3 and for regression analysis with Firth bias correction package ‘logistf’ version 1.24.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Participant characteristics
Data from 91 participants (38 young children, 53 adults) was included in the analyses. Six participants had to be excluded due to detectable antibodies at the baseline sampling: for four participants, the questionnaire data indicated that these persons were vaccinated against COVID-19. For the other two participants, no information was given in the questionnaire about a previous infection. We assume that these participants had an undetected SARS-CoV-2 infection in the past.
The basic characteristics of the study population (total) and the seropositive subgroup are described in Table 1.
More than 90% of both young children and adults had a positive PCR test during the study period. Five cases were reported as positive by national health authorities using official PCR testing, but possessed negative PCR test results during study sampling afterwards.
In those cases, no Ct values were available. Viral load and interval between the probable date of infection and antibody testing at follow-up were comparable between adults and young children. Symptoms were more frequent in adults than in young children, both in the entire cohort and in the seropositive subgroup.
Seroconversion findings
The seroconversion rate in young children was significantly higher than in adults: 97.4% (n = 37) of the young children and 66% (n = 35) of the adults had detectable S-antigen IgG antibodies at the follow-up sampling about seven weeks after the probable date of infection (p < 0.001).
Multivariable logistic regression analysis for association between seroconversion and several determinants
For 86 of 91 study participants, regression analysis was performed (Table 2). Five cases had to be excluded due to missing Ct values. Seroconversion was associated with viral load (Ct value), time interval between the probable date of infection and antibody testing at follow-up, and age (seroconverted person being an adult). The higher the viral load (i.e., a lower Ct value) and the longer the time interval between the probable date of infection and antibody testing, the more likely it was for participants to show seroconversion. The indicator variable for age (the person tested being an adult) showed that participants aged over 18 years were less likely to seroconvert.
Discussion
Ninety-seven percent of young children, versus 66% of adults, had developed SARS-CoV-2 antibodies at day 43-56 after the date of probable infection. The difference in seroconversion rates between young children and adults was statistically significant. Independent of age, a high viral load at the time of acute infection was positively associated with seroconversion. A longer time interval between the probable date of infection and antibody testing at follow-up increased the odds of seroconversion, although the variability in time span within the entire study group was small (up to 14 days). That was to be expected, however, as we sampled all participants according to study protocol at similar times.
Interestingly, other studies found a considerably lower seroconversion rate among young children, compared to our results. Whereas 97% of young children in our study had developed SARS-CoV-2 antibodies, Toh et al. detected antibodies in only 37%9, and Ruedas-Lopez et al. in 37.5%12 of tested children. One explanation for the substantial differences to our study results may be the level of viral load. Young children in our study population had relatively high viral loads (median Ct value of 26). In accordance with our results, Toh et al. concluded from a subgroup of their study sample that high viral loads (Ct < 26) may be associated with higher seroconversion rates9. Also, Walker et al. found that participants (≥16 years) with higher viral load (Ct < 25) showed seroconversion significantly more frequently than participants with lower viral load (Ct > 25)24.
A positive association between high viral load and seroconversion, regardless of age, was also shown in our analysis. We hypothesize that young children were more likely to have developed antibodies following a SARS-CoV-2 infection than adults due to their fast and strong immune system20,25.
Our findings on seroconversion in adults (66%) are comparable with seroconversion rates also assessed by S-antigen antibodies against SARS-CoV-2 reported in literature ranging from 57.7% to 76.6% to 90%9,26,27.
With respect to the time interval between the probable date of infection and antibody testing at follow-up, we can assume that we have captured the seroconversion of all individuals. As studies have shown that IgG antibodies against spike protein are reliably detectable in blood samples after more than 14 days and up to 4 to 5 months after the first positive PCR or symptom onset3,4,6,7,28,29.
Strengths of our study are the assessment of young children in a non-clinical, community setting, the prospective study design including the collection of biological samples (repeated PCR tests, antibody testing at two points of time), and close monitoring of symptoms.
Nevertheless, some limitations should be considered. Our study has a relatively small sample size that limits statistical power. However, comparable studies have similarly small numbers of participants, presumably due to the young age of the study subjects8,9,12. Although participants were selected in the context of SARS-CoV-2 outbreaks in daycare centers all over Germany, the sample of daycare centers was not randomized and thus may not be representative. Overall, 59% of the invited young children and adults of a daycare group and their close contacts participated. Reasons for non-participation were not evaluated.
Furthermore, the results of the indicator variable suggest that young children seroconvert more frequently than adults. However, the number of cases in our study is too small to estimate this precisely. Therefore, we do not discuss age as an associating factor for seroconversion.
Concerning the laboratory testing of antibodies, the results are semi-quantitative. Since the aim of the study was to investigate seroconversion, not the strength of the immune response, we did not perform quantitative measurements of the antibody concentrations. Overall, it is possible that under-reporting of infected individuals may have occurred due to false-negative PCR samples. Prolonged unrefrigerated transport in hot temperatures can lead to false-negative PCR results. However, the majority of our samples were collected in winter and spring. We, therefore, consider under-reporting, in the sense of an increased number of false-negative samples, to be very unlikely. In addition, all samples arrived at the laboratory within the time frame specified by the study.
Viral variants of SARS-CoV-2 may induce different immunological responses. Our findings are derived from the ancestral and alpha SARS-CoV-2 variants that were predominant at the time of data collection in 2020/2021 in Germany. The results may therefore be not transferable to subsequent variants. Recent analyses in Australian children after infection with Delta- or Omicron SARS-CoV-2 variants show that seroconversion rates in young children (6 months-17 years; median 3–4 years of age) one month after a positive PCR were 100% for the Delta variant (n = 35/35 children) and 81% for the Omicron variant (n = 13/16 children)14. These results are compatible with ours but differ from previously reported results for this age group.
Our findings contribute to the overall picture of published but still scarce data on seroconversion after infection with SARS-CoV-2 in young children.
Data availability
The datasets presented in this article are not readily available because the authors confirm that some access restrictions apply to the data underlying the findings. The dataset cannot be made publicly available because informed consent from study participants did not cover public deposition of data. However, the source dataset underlying the findings is archived in the “Health Monitoring” Research Data Centre at the Robert Koch Institute (RKI) and can be accessed by researchers on reasonable request. On-site access to the dataset is possible at the Secure Data Center of the RKI’s “Health Monitoring” Research Data Center. Requests to access the datasets should be directed to “Health Monitoring” Research Data Center, Robert Koch Institute, Berlin, Germany (fdz@rki.de).
References
Sun, B. et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 9, 940–948 (2020).
Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020).
Okba, N. M. A. et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 26, 1478–1488 (2020).
Zhao, J. et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 71, 2027–2034 (2020).
Calitri, C. et al. Long-term clinical and serological follow-up of paediatric patients infected by SARS-CoV-2. Infez. Med. 29, 216–223 (2021).
Lazzerini, M. et al. Evolution of SARS-CoV-2 IgG seroprevalence in children and factors associated with seroconversion: results from a multiple time-points study in Friuli-Venezia Giulia Region, Italy. Children 9, 246 (2022).
Oygar, P. D. et al. Longitudinal follow-up of antibody responses in pediatric patients with COVID-19 up to 9 months after infection. Pediatr. Infect. Dis. J. 40, e294–e299 (2021).
Callejas-Caballero, I. et al. A prospective study of the serological, clinical, and epidemiological features of a SARS-CoV-2 positive pediatric cohort. Children (Basel, Switzerland) 9, 665 (2022).
Toh, Z. Q. et al. Comparison of seroconversion in children and adults with mild COVID-19. JAMA Network Open 5, e221313–e221313 (2022).
Ständige Impfkommission: Beschluss der STIKO zur 15. Aktualisierung der COVID-19-Impfempfehlung. Epidemiol. Bull. 3–15 https://doi.org/10.25646/9437 (2022).
Ständige Impfkommission: Beschluss der STIKO zur 23. Aktualisierung der COVID-19-Impfempfehlung. Epidemiol. Bull. 3–21 https://doi.org/10.25646/10788 (2022).
Ruedas-Lopez, A. et al. Longitudinal survey of humoral and cellular response to SARS-CoV-2 infection in children. Microbiol. Res. 264, 127145 (2022).
Lorthe, E. et al. Epidemiological, virological and serological investigation of a SARS-CoV-2 outbreak (Alpha variant) in a primary school: a prospective longitudinal study. PLoS ONE 17, e0272663 (2022).
Toh, Z. Q. et al. Comparison of antibody responses to SARS-CoV-2 variants in Australian children. Nat. Commun. 13, 7185 (2022).
Schienkiewitz, A. et al. SARS-CoV-2 transmissibility within day care centers-study protocol of a prospective analysis of outbreaks in Germany. Front. Public Health 9, 773850 (2021).
Wurm, J. et al. Symptomatik einer akuten SARS-CoV-2-Infektion bei Kindern im Kita-Alter. Monatsschrift Kinderheilkunde, 170, 1113–1121 (2022).
Robert Koch-Institut. Application of the Euroimmun anti-SARS-CoV-2-S1-IgG ELISA Antibody Test to Dried Blood Spots. (Robert Koch-Institut, 2021).
Michel, J. et al. Resource-efficient internally controlled in-house real-time PCR detection of SARS-CoV-2. Virol. J. 18, 110 (2021).
Loss, J. et al. Transmission of SARS-CoV-2 among children and staff in German daycare centres. Epidemiol. Infect. 150, e141 (2022).
Zimmermann, P. & Curtis, N. Why does the severity of COVID-19 differ with age?: understanding the mechanisms underlying the age gradient in outcome following SARS-CoV-2 infection. Pediatr. Infect. Dis. J. 41, e36–e45 (2022).
Wiedenmann, M. et al. The role of children and adolescents in the SARS-CoV-2 pandemic: a rapid review. Swiss Med. Wkly 151, w30058 (2021).
Sandoni, A. et al. SARS-CoV-2 viral clearance and viral load kinetics in young children (1–6 years) compared to adults: results of a longitudinal study in Germany. Front. Pediatr. 10, 989456 (2022).
Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38 (1993).
Walker, A. S. et al. Ct threshold values, a proxy for viral load in community SARS-CoV-2 cases, demonstrate wide variation across populations and over time. Elife 10, e64683 (2021).
Zimmermann, P. & Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. https://doi.org/10.1136/archdischild-2020-320338 (2020).
Renk, H. et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat. Commun. 13, 128 (2022).
Schuler, C. F. T. et al. Mild SARS-CoV-2 illness is not associated with reinfections and provides persistent spike, nucleocapsid, and virus-neutralizing antibodies. Microbiol. Spectr. 9, e0008721 (2021).
Wajnberg, A. et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370, 1227–1230 (2020).
Gudbjartsson, D. F. et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl J. Med. 383, 1724–1734 (2020).
Kuger, S. et al. Die Kindertagesbetreuung während der COVID-19-Pandemie. Ergebnisse einer Interdisziplinären Studie. (wbv Publikation, 2022).
Acknowledgements
Corona-outbreak-related examinations in daycare centers—COALA was conducted by the RKI and was funded by the Federal Ministry of Health under grant number ZMVI1-2520COR404 for the period June 2020 to December 2021. COALA was one out of four modules of the “Corona daycare center study: Research on the organizational, hygienic and educational challenges of emergency daycare in daycare centers as well as on acute respiratory diseases during the implementation of measures to contain SARS-CoV-2”, which was conducted by the German Youth Institute (DJI) together with the RKI. The Federal Ministry of Health was not involved in the design of the study or collection of data. We would like to thank our colleagues at the RKI in the Department Epidemiology and Health Monitoring, as well as the Department Infectious Disease Epidemiology, for their co-operation and support of our study. We are especially indebted to Udo Buchholz and Professor Walter Haas, who contributed to the design of the study and were valued partners in discussing the results. We also want to thank Angelika Schaffrath Rosario for her advice on data analyses. We thank our colleagues of SurvAd and the COALA study team at the RKI for their support in conducting the study as well as our colleagues at the Epidemiological Central Laboratory for processing the samples. We would also like to thank all enrolled children, parents and daycare staff for their participation in the study. A brief, descriptive presentation of the results on seroconversion of children and adults in COALA is available in German language in the final national report on the study. This final report was requested by the funding body (Federal Ministry of Health) and was published in November 202230.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.L., A. Schienkiewitz, and S.J. designed the study. J.W., A. Sandoni, U.K., and B.F. were responsible for participant recruitment, data and specimen collection. M.S., A. Sandoni, U.K. performed laboratory testing and analyzed lab results. U.K., M.S., J.W., T.K. carried out the analysis and were responsible for the accuracy of the data analysis. U.K., A. Sandoni, A. Schienkiewitz, and J.L. wrote the manuscript, which was then reviewed and approved by all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubisch, U., Sandoni, A., Wurm, J. et al. SARS-CoV-2 seroconversion in children attending daycare versus adults in Germany between October 2020 and June 2021. Commun Med 3, 124 (2023). https://doi.org/10.1038/s43856-023-00352-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00352-3