Abstract
Aged hematopoietic stem cells (HSCs) exhibit compromised reconstitution capacity and differentiation bias toward myeloid lineages. However, the molecular mechanism behind HSC aging remains largely unknown. In this study, we observed that RNA N1-methyladenosine-generating methyltransferase TRMT6–TRMT61A complex is increased in aged murine HSCs due to aging-declined CRL4DCAF1-mediated ubiquitination degradation signaling. Unexpectedly, no difference of tRNA N1-methyladenosine methylome is observed between young and aged hematopoietic stem and progenitor cells, suggesting a noncanonical role of the TRMT6–TRMT61A complex in the HSC aging process. Further investigation revealed that enforced TRMT6–TRMT61A impairs HSCs through 3′-tiRNA-Leu-CAG and subsequent RIPK1–RIPK3–MLKL-mediated necroptosis cascade. Deficiency of necroptosis ameliorates the self-renewal capacity of HSCs and counters the physiologically deleterious effect of enforced TRMT6–TRMT61A on HSCs. Together, our work uncovers a nonclassical role for the TRMT6–TRMT61A complex in HSC aging and highlights a therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq, m1A-seq and tRF-seq data have been deposited in the Gene Expression Omnibus under accession code GSE163301. The proteomics data have been deposited to the ProteomeXchange Consortium via the iProX partner repository64 under accession number PXD047430. All other data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
He, H. et al. Aging-induced IL27Ra signaling impairs hematopoietic stem cells. Blood 136, 183–198 (2020).
Wang, J. et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 148, 1001–1014 (2012).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M. & de Haan, G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208, 2691–2703 (2011).
Ermolaeva, M., Neri, F., Ori, A. & Rudolph, K. L. Cellular and epigenetic drivers of stem cell ageing. Nat. Rev. Mol. Cell Biol. 19, 594–610 (2018).
de Haan, G. & Lazare, S. S. Aging of hematopoietic stem cells. Blood 131, 479–487 (2018).
Delaunay, S. & Frye, M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 21, 552–559 (2019).
Guzzi, N. et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell 173, 1204–1216 (2018).
Yao, Q. J. et al. Mettl3–Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell Res. 28, 952–954 (2018).
Cheng, Y. et al. m6A RNA methylation maintains hematopoietic stem cell identity and symmetric commitment. Cell Rep. 28, 1703–1716 (2019).
Lee, H. et al. Stage-specific requirement for Mettl3-dependent m6A mRNA methylation during haematopoietic stem cell differentiation. Nat. Cell Biol. 21, 700–709 (2019).
Li, X., Xiong, X. & Yi, C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat. Methods 14, 23–31 (2017).
Dominissini, D. et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature 530, 441–446 (2016).
Li, X. et al. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat. Chem. Biol. 12, 311–316 (2016).
Li, X. et al. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68, 993–1005 (2017).
Zhou, H. et al. Evolution of a reverse transcriptase to map N1-methyladenosine in human messenger RNA. Nat. Methods 16, 1281–1288 (2019).
Xiong, X., Li, X. & Yi, C. N1-methyladenosine methylome in messenger RNA and non-coding RNA. Curr. Opin. Chem. Biol. 45, 179–186 (2018).
Ozanick, S., Krecic, A., Andersland, J. & Anderson, J. T. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA 11, 1281–1290 (2005).
Safra, M. et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551, 251–255 (2017).
Finer-Moore, J., Czudnochowski, N., O’Connell, J. D., Wang, A. L. & Stroud, R. M. Crystal structure of the human tRNA m1A58 methyltransferase–tRNA3Lys complex: refolding of substrate tRNA allows access to the methylation target. J. Mol. Biol. 427, 3862–3876 (2015).
Su, Z., Wilson, B., Kumar, P. & Dutta, A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu. Rev. Genet. 54, 47–69 (2020).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Shan, B., Pan, H., Najafov, A. & Yuan, J. Necroptosis in development and diseases. Genes Dev. 32, 327–340 (2018).
Gonzalez-Juarbe, N. et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1005337 (2015).
Mocarski, E. S., Guo, H. Y. & Kaiser, W. J. Necroptosis: the Trojan horse in cell autonomous antiviral host defense. Virology 479, 160–166 (2015).
Caccamo, A. et al. Necroptosis activation in Alzheimer’s disease. Nat. Neurosci. 20, 1236–1246 (2017).
Ito, Y. et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353, 603–608 (2016).
Zhang, T. et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 22, 175–182 (2016).
Strilic, B. et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536, 215–218 (2016).
Li, D. et al. RIPK1-RIPK3-MLKL-dependent necrosis promotes the aging of mouse male reproductive system. eLife 6, e27692 (2017).
Yamashita, M. & Passegué, E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell 25, 357–372 (2019).
Zhang, X. et al. YTHDF3 modulates hematopoietic stem cells by recognizing RNA m6A modification on Ccnd1. Haematologica 107, 2381–2394 (2022).
Git, A. et al. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 16, 991–1006 (2010).
Mestdagh, P. et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nat. Methods 11, 809–815 (2014).
Bertheloot, D., Latz, E. & Franklin, B. S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cellular Mol. Immunol. 18, 1106–1121 (2021).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Ren, Y. et al. Discovery of a highly potent, selective, and metabolically stable inhibitor of receptor-interacting protein 1 (RIP1) for the treatment of systemic inflammatory response syndrome. J. Med. Chem. 60, 972–986 (2017).
Caserta, T. M., Smith, A. N., Gultice, A. D., Reedy, M. A. & Brown, T. L. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8, 345–352 (2003).
Liu, M., Li, H., Yang, R., Ji, D. & Xia, X. GSK872 and necrostatin-1 protect retinal ganglion cells against necroptosis through inhibition of RIP1/RIP3/MLKL pathway in glutamate-induced retinal excitotoxic model of glaucoma. J. Neuroinflammation 19, 262 (2022).
Liu, Y. et al. RIP1 kinase activity-dependent roles in embryonic development of Fadd-deficient mice. Cell Death Differ. 24, 1459–1469 (2017).
Li, D. et al. RIPK1-RIPK3-MLKL-dependent necrosis promotes the aging of mouse male reproductive system. eLife https://doi.org/10.7554/eLife.27692 (2017).
Zhang, L. et al. Ripk3 signaling regulates HSCs during stress and represses radiation-induced leukemia in mice. Stem Cell Rep. 17, 1428–1441 (2022).
Newton, K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 25, 347–353 (2015).
Platt, R. J. et al. CRISPR–Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Jackson, S. & Xiong, Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem. Sci. 34, 562–570 (2009).
Pietras, E. M. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood 130, 1693–1698 (2017).
Higa, K. C. et al. Chronic interleukin-1 exposure triggers selection for Cebpa-knockout multipotent hematopoietic progenitors. J. Exp. Med. https://doi.org/10.1084/jem.20200560 (2021).
Frank, D. & Vince, J. E. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Diff. 26, 99–114 (2019).
Yang, X., Cong, T., He, H. & Wang, J. GSDME maintains hematopoietic stem cells by balancing pyroptosis and apoptosis. Blood Sci. 3, 40–47 (2021).
Tao, P. et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114 (2020).
Wang, R. et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 580, 386–390 (2020).
Jiao, H. et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 (2020).
Becker, F. & Rudolph, K. L. Targeting enzyme aging. Science 371, 462–463 (2021).
Yamasaki, S., Ivanov, P., Hu, G.-F. & Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 185, 35–42 (2009).
Goncalves, K. A. et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell 166, 894–906 (2016).
Silberstein, L. et al. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell 19, 530–543 (2016).
Schimmel, P. The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 19, 45–58 (2018).
Kim, H. K. et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 552, 57–62 (2017).
Gao, J. et al. The CUL4–DDB1 ubiquitin ligase complex controls adult and embryonic stem cell differentiation and homeostasis. eLife https://doi.org/10.7554/eLife.07539 (2015).
Guo, Z. et al. DCAF1 regulates Treg senescence via the ROS axis during immunological aging. J. Clin. Invest. 130, 5893–5908 (2020).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R Package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2021).
Acknowledgements
We thank X. Wang (National Institute of Biological Sciences) for providing Ripk3−/− and Mlkl−/− mice and H. Zhang (Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for providing Ripk1K45A mice. We thank Z. Zhang (National Institute of Biological Sciences) for providing RIPA-56. We thank Z. Xu (Zhejiang University School of Medicine) for providing mouse ANG and anti-ANG. We thank the Beijing Advanced Innovation Center for Structural Biology and the Tsinghua-Peking Center for Life Sciences for facility and financial support. This work was supported by grant numbers 82250002, 92249305, Z200022 and 2018YFA0800200 to J.W. from the National Key R&D Program of China or the Beijing Municipal Science & Technology Commission and the National Natural Science Foundation of China. This work was also supported by the National Key R&D Program of China (nos. 2019YFA0802200 and 2019YFA0110900) and the National Natural Science Foundation of China (nos. 91940304, 31861143026 and 21825701 to C.Y.).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.W. and C.Y. Methodology: H.H., Y.W., X.Z. and X.L. Investigation: H.H., Y.W., X.Z., X.L., C.L., D.Y., H.D., W.S., C.Y. and J.W. Formal analysis: J.W., C.Y., H.H. and X.Z. Resources: J.W. and C.Y. Writing: J.W. and C.Y. Funding acquisition: J.W. and C.Y. Supervision: J.W. and C.Y.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Philipp Jost and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Enforced Trmt6/61a mildly disturbs the homeostasis of hematopoietic system at young age.
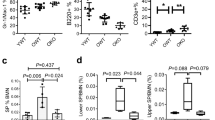
(a) The schematic diagram showing the strategy to generate SFFV-Trmt6/61a-transgenic mice. (b-c) 3-month old Trmt6/61aTG or WT mice were analyzed for white blood cell (WBC), lymphocyte (LYM), neutrophil (NEUT), red blood cell (RBC), platelet (PLT), myeloid, B cells, and T cells. (b) The scatter plots show the count of WBC, LYM, NEUT, RBC and PLT between Trmt6/61aTG and WT mice. (c) The scatter plots depict the percentage of B, CD4+ T, CD8+ T cells, and myeloid in peripheral blood. n = 12 mice per group. (d-h) 8−week-old Trmt6/61aTG and age-matched WT mice were analyzed for analyzed for myeloid, B cells, T cells, progenitors and HSCs. (d) Percentage of myeloid, B, CD4+ T and CD8+ T cells in bone marrow. (e and f) This histogram shows the frequency (e) and the number per femur (f) of CMP, GMP, MEP, and CLP between Trmt6/61aTG and WT mice. (g and h) This histogram shows the frequency (g) and the number per femur (h) of LT-HSC, ST-HSC and MPP between Trmt6/61aTG and WT mice. The surface marker combinations are listed in Supplementary Table 3. n = 8 WT mice and n = 6 Trmt6/61aTG mice. (i-m) 30 HSCs from either 2-month old Trmt6/61aTG mice or age-matched WT mice were transplanted into lethally irradiated recipients together with 2×105 competitor cells. Chimera in peripheral blood and bone marrow was checked every month until the 3rd month. (i) Percentage of donor cell reconstitution in overall (CD45.2+), B (B220+), T (CD3+) and myeloid (Mac-1+) cell every month after transplantation. (j and l) The lineage distribution in donor-derived peripheral blood (j) and bone marrow (l) at the 3rd month. (k and m) Percentage of donor cell reconstitution in bone marrow (k) and HSC (m). n = 9-10 mice per group (WT: n = 9; Trmt6/61aTG: n = 10). Two-tailed unpaired Student’s t-test was used for statistical analysis in b and i. Data are shown as the mean ± SD in c-m.
Extended Data Fig. 2 Aged HSCs exhibit altered tRF profile compared to young ones.
(a) This line plots showing the tRF reads length distribution between young and aged KSL cells. (b) This histogram shows the percentage of the indicated tRF types between young and aged KSL cells. (c) Volcano Plot showing the expression of tRF-5, tRF-3, 5’-tRNA half (5’-tiRNA), 3′ -tRNA half (3’-tiRNA), and internal-tRF between young and aged KSL cells. (d) The scatterplot depicts the tRNA expression in young and aged KSL cells. (e) The histogram showing the expression of tRNA-derived fragments between young and aged KSL cells. The sequence of individual tRF was listed in Table S4. n = 3 independent experiments. (f) The histogram displays the expression of 3’-tiRNA-Leu-CAG in KSL cells carrying indicated shRNA. n = 3 independent experiments. Two-tailed unpaired Student’s t-test was used for statistical analysis in e-f. Data are shown as the mean ± SD in e-f.
Extended Data Fig. 3 ANG associates with the TRMT6/61A complex primarily through TRMT61A.
(a) The schematic diagram (left) showing the experimental design to evaluate the influence of ANG on the expression of 3’-tiRNA-Leu-CAG in KSL cells. The histogram (right) displays the alteration of 3’-tiRNA-Leu-CAG in response to ANG treatment in KSL cells. n = 3 independent experiments. Two-tailed unpaired Student’s t-test was used for statistical analysis. Data are shown as the mean ± SD. (b) Pulldown assay shows the directly interaction between recombinant ANG and His-TRMT61A purified from E. coli. Ni-NTA beads serves as negative control for ANG binding. (Upper panel) ANG was detected by immunoblotting. (Lower panel) Purified proteins were visualized by Coomassie blue staining. (c) Purified His-TRMT6/61A were incubated with recombinant ANG in the presence or absence of purified yeast tRNA (“+”: 2 μg/mL; “+++”: 20 μg/mL) at 4 °C for 1 hr. Pulldown assay shows the interaction between ANG and TRMT6/61A was independent of tRNA. Purified proteins were either detected by immunoblotting or visualized by Coomassie staining. (d) The schematic diagram displays the strategy to generate the various truncations of TRMT61A. (e) HEK293T cells were transiently transfected with Myc-tagged wild-type or various truncations of TRMT61A together with SFB-tagged ANG. 24 hours later, cell lysates were immunoprecipitated with S beads, and western blot analysis was performed with indicated antibodies. Representative western blot shows the central fragment (amino acids 90-183) of TRMT61A is required for ANG binding.
Extended Data Fig. 4 Necroptosis is the target of 3’-tiRNA-Leu-CAG to regulate HSC function.
(a) Representative western blot showing the expression of GSDMD, GSDME, TRMT6 and TRMT61A in Trmt6/61aTG and WT KSL cells. Asterisk, non-specific band. (b-c) Experimental design (b). (c) Representative western blot showing the expression of RIPK1, RIPK3, MLKL, TRMT6 and TRMT61A in TRMT6/61A-carrying lentivirus infected KSL cells. Asterisk, non-specific band. (d) Representative western blot showing the expression of RIPK1, RIPK3, MLKL, TRMT6 and TRMT61A in KSL cells carrying indicated shRNA. (e and f) Freshly isolated KSL cells were infected by TRMT6/61A-carrying lentivirus and 24 hours later, these cells were transfected with Anti-3’-tiRNA-Leu and Anti-5’-tiRNA-Leu (control) respectively. Another 48 hours later, FACS-purified GFP+ cells were seeded to recover for 24 hours, and then were subjected to Western Blot to detect necroptotic proteins. (e) Experimental design. (f) Representative western blot showing the expression of RIPK1, RIPK3, TRMT6 and TRMT61A. (g) The mRNA expression of Ripk1 and Ripk3 in Trmt6/61aTG and WT KSL cells. n = 3 independent experiments. (h) The mRNA expression of Ripk1 and Ripk3 in in 3’-tiRNA-Leu-CAG transfected KSL cells. n = 6 independent experiments. (i and j) Protein synthesis rates were determined by OP-Puro incorporation in Trmt6/61aTG and WT KSL cells. (i) Representative flow cytometry. (j) The scatterplot depicts the relative protein synthesis rates in Trmt6/61aTG and WT KSL cells after 1 hour labeling. n = 3 independent experiments. (k) This histogram depicts the percentage of ribosome occupancy of Ripk1 and Ripk3 mRNAs measured by real-time PCR. After sucrose gradient fractionation of polyribosomes, the relative ratio of translated mRNA was measured by normalizing polyribosome mRNA to the input mRNA. n = 3 independent experiments. (l) The histogram displays the relative distribution of 3’-tiRNA-Leu-CAG in fraction pools identified as free-monosomes (FM) and polysomes. After sucrose gradient fractionation of polyribosomes, the relative ratio of 3’-tiRNA-Leu-CAG was measured using real-time PCR by normalizing to U6 small nuclear RNA. n = 3 independent experiments. Two-tailed unpaired Student’s t-test was used for statistical analysis in k-l. Data are shown as the mean ± SD in g,h and j-l.
Extended Data Fig. 5 Targeted dysfunction of necroptosis signaling ameliorates HSCs.
(a-b) 10 HSCs from 5-month old Ripk1K45A or WT mice were transplanted into recipients together with 3×105 competitor cells. (a) Percentage of donor cell reconstitution in overall, B, T and myeloid cell every month after transplantation. (b) The lineage distribution of donor-derived peripheral blood at the 3rd month. n = 5 mice per group. (c-d) Secondary transplantation assay for Ripk1K45A HSC. (c) Percentage of donor cell reconstitution in overall, B, T and myeloid cell every month after transplantation. (d) The lineage distribution of donor-derived peripheral blood at the 3rd month. n = 6-7 mice per group (WT: n = 6; Ripk1K45A: n = 7). (e-f) 20 HSCs from 2 months old Ripk3-/- or WT mice were transplanted into recipients together with 6×105 competitor cells. (e) Percentage of donor cell reconstitution in overall, B, Tand myeloid cell every month after transplantation. (f) The lineage distribution of donor-derived peripheral blood at the fourth month. n = 6-7 mice per group (WT: n = 6; Ripk3-/-: n = 7). (g-h) 20 HSCs from either 5-month old Mlkl-/- or WT mice were transplanted into recipients together with 3×105 competitor cells. (g) Percentage of donor cell reconstitution in overall, B, T and myeloid cell every month after transplantation. (h) The lineage distribution of donor-derived peripheral blood at the 4th month. n = 5-6 mice per group (WT: n = 5; Mlkl-/-: n = 6). (i) 20 HSCs from 5 months old Mlkl-/- or WT mice were transplanted into recipients together with 3×105 competitor cells. Four months after, two million bone marrow cells of primary recipients were transplanted into secondary recipients. Three months after, 50 HSCs isolated from secondary recipients were transplanted into the tertiary recipients together with 3×105 competitor cells. These line plots display the percentage of donor cell reconstitution in overall, B, T and myeloid cell every month after transplantation. n = 5-6 mice per group (WT: n = 6; Mlkl-/-: n = 5). Two-tailed unpaired Student’s t-test was used for statistical analysis in c, e and i. Data are shown as the mean ± SD in a-i.
Extended Data Fig. 6 Trmt6/Trmt61a signaling is essential for HSC maintenance.
(a) The schematic diagram showing the targeting strategy to generate Trmt6-/- mice. (b) The schematic diagram showing the targeting strategy to generate Trmt61a-/- mice. (c and d) Freshly isolated KSL cells either from young mice (3 months) or aged mice (18 months) were infected with shTrmt6 and shTrmt61a, and 72 hours later, 100 GFP+ CD48- Sca1+ cells were FACS-purified and subsequently cultured in SFEM medium and then evaluated their proliferation potential via colony size observation and the cell number enumeration on day 7. (c) Representative images of ex vivo cultured HSC carrying the indicated shRNA on day 7. Scale bar, 50 μm. (d) The scatter plots depict the cell numbers of ex vivo cultured HSC carrying the indicated shRNA on day 7. n = 8-10 independent experiments (n = 8 for shCon, n = 10 for shTrmt6 and shTRMT61a). (e and f) 32D cells stably expressing wild-type TRMT6/61A or the catalytic dead mutant were infected with lentiviral shRNA constructs that target the 3’ UTR of Trmt6 and Trmt61a. The cells were used to detect the indicated proteins (e), (f) 50 cells were FACS-purified and subsequently cultured in IL-3 containing 1640 medium, the cell number were enumeration on day 6. Asterisk, non-specific band. n = 7 independent experiments. Two-tailed unpaired Student’s t-test was used for statistical analysis in d and f. Data are shown as the mean ± SD in d and f.
Extended Data Fig. 7 Working model.
(a) This chart illustrates the proposed model of TRMT6/61A→3’-tiRNA-Leu-CAG →RIPK1→RIPK3→MLKL cascade promotes hematopoietic stem cell aging. (b) This chart illustrates the summary model of the connection between molecular mechanisms and cellular phenotypes.
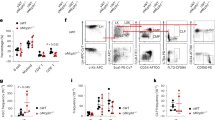
Extended Data Fig. 8 Representative flow cytometry gating strategy used in this study.
(a) Representative flow cytometry showing the gating strategy used to analyze and isolate the hematopoietic stem and progenitor cell populations in mouse bone marrow presented on Figs. 1a-b, e, h, 2b-c, e-m, 3a-c, d, f, i, j, m, 4a, f-g, i-o, 5a, d, 6f-i, 7h, i, and Extended Data Figs. 1e-h, 2a-f, 3a, 4a-l, 6c-d. (b) Strategy used to evaluate the the percentage of B, myeloid, CD4+ T, CD8+ T cells presented on Fig. 1j and Extended Data Fig. 1c-d. (c-d) Strategy used to evaluate the the percentage (c) and lineage distribution (d) of test donor-derived cells (myeloid, B and T cells) presented on Figs. 1g, k-l, 2d, 3h, 5b, e-k, l-m, 6d-e and Extended Data Figs. 1i-j, 5a-i.
Supplementary information
Supplementary Information
Supplementary Methods and repeated western blots.
Supplementary Tables 1–4
Supplementary Table 1. GSEA gene list; Supplementary Table 2. Oligonucleotides; Supplementary Table 3. Materials; Supplementary Table 4. tRFs sequence.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blot.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blot.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blot.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blot.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blot.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, H., Wang, Y., Zhang, X. et al. Age-related noncanonical TRMT6–TRMT61A signaling impairs hematopoietic stem cells. Nat Aging 4, 213–230 (2024). https://doi.org/10.1038/s43587-023-00556-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00556-1