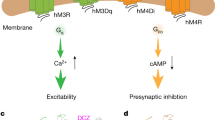
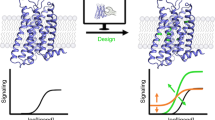
Abstract
Chemogenetics is an approach for engineering proteins to enable their modulation by otherwise inert small molecules. Although kinases, enzymes and ion channels have been used for chemogenetics, the most widely used platform is based on G protein-coupled receptors (GPCRs), using designer receptors exclusively activated by designer drugs (DREADDs). DREADDs have been used ubiquitously to modulate cellular signalling and neuronal activity and are a key technology for modern causal neuroscience. Here we provide a Primer on key aspects of DREADD technology, emphasizing how to reliably design and validate chemogenetic transducers and actuators. We also provide recommendations for the use of DREADDs for specialized applications including modulating metabolically essential peripheral tissues and distinct neuronal populations in non-human primates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hopkins, A. L. & Groom, C. R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Wacker, D., Stevens, R. C. & Roth, B. L. How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–427 (2017).
Roth, B. L. Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs. Nat. Struct. Mol. Biol. 26, 535–544 (2019).
Regard, J. B., Sato, I. T. & Coughlin, S. R. Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 (2008).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000).
Reisberg, B. et al. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 348, 1333–1341 (2003).
Errington, A. C., Stohr, T. & Lees, G. Voltage gated ion channels: targets for anticonvulsant drugs. Curr. Top. Med. Chem. 5, 15–30 (2005).
Elkins, J. M. et al. Comprehensive characterization of the Published Kinase Inhibitor Set. Nat. Biotechnol. 34, 95–103 (2016).
Changeux, J. P. & Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell 166, 1084–1102 (2016).
Keiser, M. J. et al. Predicting new molecular targets for known drugs. Nature 462, 175–181 (2009).
Besnard, J. et al. Automated design of ligands to polypharmacological profiles. Nature 492, 215–220 (2012).
Roth, B. L., Sheffler, D. J. & Kroeze, W. K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 3, 353–359 (2004).
Yadav, P. N. et al. The presynaptic component of the serotonergic system is required for clozapine’s efficacy. Neuropsychopharmacology 36, 638–651 (2011).
Cao, C. et al. Structure, function and pharmacology of human itch GPCRs. Nature 600, 170–175 (2021).
Keiser, M. et al. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 25, 197–206 (2007).
McGovern, S. L. & Shoichet, B. K. Kinase inhibitors: not just for kinases anymore. J. Med. Chem. 46, 1478–1483 (2003).
Anastassiadis, T., Deacon, S. W., Devarajan, K., Ma, H. & Peterson, J. R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1039–1045 (2011).
Arrowsmith, C. H. et al. The promise and peril of chemical probes. Nat. Chem. Biol. 11, 536–541 (2015).
Strader, C. D. et al. Allele-specific activation of genetically engineered receptors. J. Biol. Chem. 266, 5–8 (1991).
Coward, P. et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl Acad. Sci. USA 95, 352–357 (1998).
Westkaemper, R. et al. Engineering in a region of bulk tolerance into the 5-HT2A receptor. Eur. J. Med. Chem. 34, 441–447 (1999).
Bishop, A. C. et al. Design of allele-specific inhibitors to probe protein kinase signaling. Curr. Biol. 8, 257–266 (1998).
Liu, Y., Shah, K., Yang, F., Witucki, L. & Shokat, K. M. Engineering Src family protein kinases with unnatural nucleotide specificity. Chem. Biol. 5, 91–101 (1998).
Bishop, A. C. et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 (2000).
Lopez, M. S. et al. Staurosporine-derived inhibitors broaden the scope of analog-sensitive kinase technology. J. Am. Chem. Soc. 135, 18153–18159 (2013).
Lerchner, W. et al. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl-channel. Neuron 54, 35–49 (2007).
Magnus, C. J. et al. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333, 1292–1296 (2011).
Magnus, C. J. et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 364, eaav5282 (2019).
Weir, G. A. et al. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain 140, 2570–2585 (2017).
Raper, J. et al. Characterization of ultrapotent chemogenetic ligands for research applications in nonhuman primates. ACS Chem. Neurosci. 13, 3118–3125 (2022).
Armbruster, B. & Roth, B. Creation of designer biogenic amine receptors via directed molecular evolution. Neuropsychopharmacology 30, S265 (2005).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Bolognini, D. et al. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Nat. Chem. Biol. 15, 489–498 (2019).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Farrell, M. S. et al. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38, 854–862 (2013).
Agulhon, C. et al. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J. Physiol. 591, 5599–5609 (2013).
Grace, P. M. et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA 113, E3441–E3450 (2016).
Meister, J., Wang, L., Pydi, S. P. & Wess, J. Chemogenetic approaches to identify metabolically important GPCR signaling pathways: therapeutic implications. J. Neurochem. 158, 603–620 (2021).
Inoue, A. et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 177, 1933–1947 e1925 (2019).
Nakajima, K. I. & Wess, J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 82, 575–582 (2012).
Zhang, S. et al. Molecular basis for selective activation of DREADD-based chemogenetics. Nature 612, 354–362 (2022).
Nagai, Y. et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 23, 1157–1167 (2020).
Thompson, K. J. et al. DREADD agonist 21 is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol. Transl. Sci. 14, 61–72 (2018).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Levey, A. I., Edmunds, S. M., Koliatsos, V., Wiley, R. G. & Heilman, C. J. Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J. Neurosci. 15, 4077–4092 (1995).
Levey, A. I., Edmunds, S. M., Hersch, S. M., Wiley, R. G. & Heilman, C. J. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J. Comp. Neurol. 351, 339–356 (1995).
Hohmann, C. F., Potter, E. D. & Levey, A. I. Development of muscarinic receptor subtypes in the forebrain of the mouse. J. Comp. Neurol. 358, 88–101 (1995).
Hersch, S. M. & Levey, A. I. Diverse pre- and post-synaptic expression of m1–m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 56, 931–938 (1995).
Brann, M. R. et al. Studies of the pharmacology, localization, and structure of muscarinic acetylcholine receptors. Ann. N. Y. Acad. Sci. 707, 225–236 (1993).
Hudson, B. D. et al. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. FASEB J. 26, 4951–4965 (2012).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Ferguson, S. M. et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci. 14, 22–24 (2011).
Marchant, N. J. et al. Behavioral and physiological effects of a novel kappa-opioid receptor-based DREADD in rats. Neuropsychopharmacology 41, 402–409 (2016).
Liu, X. et al. Epac2 in midbrain dopamine neurons contributes to cocaine reinforcement via enhancement of dopamine release. eLife 11, e80747 (2022).
Cheng, D. et al. Noradrenergic consolidation of social recognition memory is mediated by beta-arrestin-biased signaling in the mouse prefrontal cortex. Commun. Biol. 5, 1097 (2022).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020).
Bender, D., Holschbach, M. & Stocklin, G. Synthesis of n.c.a. carbon-11 labelled clozapine and its major metabolite clozapine-N-oxide and comparison of their biodistribution in mice. Nucl. Med. Biol. 21, 921–925 (1994).
Jann, M. W., Lam, Y. W. & Chang, W. H. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch. Int. Pharmacodyn. Ther. 328, 243–250 (1994).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Urban, D. J. et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41, 1404–1415 (2016).
Zhu, H. et al. Designer receptors exclusively activated by designer drugs mice.Genesis 54, 439–446 (2016).
Roth, B. et al. A potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl Acad. Sci. USA 99, 11934–11939 (2002).
Kroeze, W. K. et al. PRESTO-TANGO as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–U328 (2015).
Schinkel, A. H. et al. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur. J. Cancer 31A, 1295–1298 (1995).
Gottesman, M. M., Pastan, I. & Ambudkar, S. V. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 6, 610–617 (1996).
Mason, B. L., Pariante, C. M. & Thomas, S. A. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology 149, 5244–5253 (2008).
Zhang, S. et al. Inactive and active state structures template selective tools for the human 5-HT5A receptor. Nat. Struct. Mol. Biol. 29, 677–687 (2022).
Wu, H. et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 485, 327–332 (2012).
Vardy, E. et al. Chemotype-selective modes of action of kappa-opioid receptor agonists. J. Biol. Chem. 288, 34470–34483 (2013).
Javitch, J. A., Ballesteros, J. A., Chen, J., Chiappa, V. & Simpson, M. M. Electrostatic and aromatic microdomains within the binding-site crevice of the D2 receptor: contributions of the second membrane-spanning segment [in process citation]. Biochemistry 38, 7961–7968 (1999).
Yan, F., Mosier, P. D., Westkaemper, R. B. & Roth, B. L. Galpha-subunits differentially alter the conformation and agonist affinity of kappa-opioid receptors. Biochemistry 47, 1567–1578 (2008).
Zhang, B., Li, L. & Lu, Q. Protein solvent-accessibility prediction by a stacked deep bidirectional recurrent neural network. Biomolecules 8, 33 (2018).
Dong, S., Rogan, S. C. & Roth, B. L. Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs. Nat. Protoc. 5, 561–573 (2010).
English, J. G. et al. VEGAS as a platform for facile directed evolution in mammalian cells. Cell 178, 748–761.e17 (2019).
Kaplan, A. L. et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 610, 582–591 (2022).
Irwin, J. J. & Shoichet, B. K. ZINC — a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 45, 177–182 (2005).
Hooker, J. M., Patel, V., Kothari, S. & Schiffer, W. K. Metabolic changes in the rodent brain after acute administration of salvinorin A. Mol. Imaging Biol. 11, 137–143 (2009).
Hooker, J. M. et al. Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology 57, 386–391 (2009).
Garner, A. R. et al. Generation of a synthetic memory trace. Science 335, 1513–1516 (2012).
Zhu, H. et al. Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory. Neuropsychopharmacology 39, 1880–1092 (2014).
Mimura, K. et al. Chemogenetic activation of nigrostriatal dopamine neurons in freely moving common marmosets. iScience 24, 103066 (2021).
Perez, P., Chavret-Reculon, E., Ravassard, P. & Bouret, S. Using inhibitory DREADDs to silence LC neurons in monkeys. Brain Sci. 12, 206 (2022).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Oguchi, M. et al. Chemogenetic inactivation reveals the inhibitory control function of the prefronto-striatal pathway in the macaque brain. Commun. Biol. 4, 1088 (2021).
Wang, L. et al. Selective activation of G(s) signaling in adipocytes causes striking metabolic improvements in mice. Mol. Metab. 27, 83–91 (2019).
Wang, L. et al. Adipocyte Gi signaling is essential for maintaining whole-body glucose homeostasis and insulin sensitivity. Nat. Commun. 11, 2995 (2020).
Caron, A. et al. Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Mol. Metab. 27, 11–21 (2019).
Zhu, L. et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5, e127994 (2019).
Liu, L. et al. Gq signaling in alpha cells is critical for maintaining euglycemia. JCI Insight 6, e152852 (2021).
Meister, J. et al. In vivo metabolic effects after acute activation of skeletal muscle G(s) signaling. Mol. Metab. 55, 101415 (2022).
Lewis, J. E. et al. Selective stimulation of colonic L cells improves metabolic outcomes in mice. Diabetologia 63, 1396–1407 (2020).
Ji, B. et al. Multimodal imaging for DREADD-expressing neurons in living brain and their application to implantation of iPSC-derived neural progenitors. J. Neurosci. 36, 11544–11558 (2016).
Bonaventura, J. et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat. Commun. 10, 4627 (2019).
Nagai, Y. et al. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat. Commun. 7, 13605 (2016).
Krishnan, S., Heer, C., Cherian, C. & Sheffield, M. E. J. Reward expectation extinction restructures and degrades CA1 spatial maps through loss of a dopaminergic reward proximity signal. Nat. Commun. 13, 6662 (2022).
Nentwig, T. B., Obray, J. D., Vaughan, D. T. & Chandler, L. J. Behavioral and slice electrophysiological assessment of DREADD ligand, deschloroclozapine (DCZ) in rats. Sci. Rep. 12, 6595 (2022).
Cerpa, J. C. et al. Inhibition of noradrenergic signalling in rodent orbitofrontal cortex impairs the updating of goal-directed actions. eLife 12, e81623 (2023).
Iwasaki, M. et al. An analgesic pathway from parvocellular oxytocin neurons to the periaqueductal gray in rats. Nat. Commun. 14, 1066 (2023).
Upright, N. A. & Baxter, M. G. Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology 45, 1793–1798 (2020).
Fujimoto, A. et al. Resting-state fMRI-based screening of deschloroclozapine in rhesus macaques predicts dosage-dependent behavioral effects. J. Neurosci. 42, 5705–5716 (2022).
Cushnie, A. K. et al. The use of chemogenetic actuator ligands in nonhuman primate DREADDs-fMRI. Curr. Res. Neurobiol. 4, 100072 (2023).
Krause, W. C. et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599, 131–135 (2021).
Claes, M. et al. Chronic chemogenetic activation of the superior colliculus in glaucomatous mice: local and retrograde molecular signature. Cells 11, 1784 (2022).
Oyama, K. et al. Chronic behavioral manipulation via orally delivered chemogenetic actuator in macaques. J. Neurosci. 42, 2552–2561 (2022).
Stachniak, T. J., Ghosh, A. & Sternson, S. M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Zheng, L., Wang, Z., Liu, Y., Zhao, J. & Huang, S. Activation of the RMTg nucleus by chemogenetic techniques alleviates the learning and memory impairment in APP/PS1 mice. Neuropsychiatric Dis. Treat. 18, 2957–2965 (2022).
Oyama, K. et al. Chemogenetic dissection of the primate prefronto-subcortical pathways for working memory and decision-making. Sci. Adv. 7, eabg4246 (2021).
Parnaudeau, S. et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77, 1151–1162 (2013).
Hayashi, T. et al. Macaques exhibit implicit gaze bias anticipating others’ false-belief-driven actions via medial prefrontal cortex. Cell Rep. 30, 4433–4444.e5 (2020).
Deffains, M. et al. In vivo electrophysiological validation of DREADD-based modulation of pallidal neurons in the non-human primate. Eur. J. Neurosci. 53, 2192–2204 (2021).
Hasegawa, T., Chiken, S., Kobayashi, K. & Nambu, A. Subthalamic nucleus stabilizes movements by reducing neural spike variability in monkey basal ganglia. Nat. Commun. 13, 2233 (2022).
Miyakawa, N. et al. Chemogenetic attenuation of cortical seizures in nonhuman primates. Nat. Commun. 14, 971 (2023).
Anacker, C. et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102 (2018).
Michaelides, M. et al. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J. Clin. Invest. 123, 5342–5350 (2013).
Casado-Sainz, A. et al. Dorsal striatal dopamine induces fronto-cortical hypoactivity and attenuates anxiety and compulsive behaviors in rats. Neuropsychopharmacology 47, 454–464 (2022).
Roelofs, T. J. M. et al. A novel approach to map induced activation of neuronal networks using chemogenetics and functional neuroimaging in rats: a proof-of-concept study on the mesocorticolimbic system. NeuroImage 156, 109–118 (2017).
Peeters, L. M. et al. Chemogenetic silencing of neurons in the mouse anterior cingulate area modulates neuronal activity and functional connectivity. NeuroImage 220, 117088 (2020).
Crawley, J. N. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice (Wiley-Liss, 2000).
Guettier, J. et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc. Natl Acad. Sci. USA 106, 19197–19202 (2009).
Li, J. H. et al. A novel experimental strategy to assess the metabolic effects of selective activation of a G(q)-coupled receptor in hepatocytes in vivo. Endocrinology 154, 3539–3551 (2013).
Kaiser, E. et al. DREADD technology reveals major impact of Gq signalling on cardiac electrophysiology. Cardiovasc. Res. 115, 1052–1066 (2019).
Akhmedov, D. et al. Gs-DREADD knock-in mice for tissue-specific, temporal stimulation of cyclic AMP signaling. Mol. Cell. Biol. 37, e00584-16 (2017).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Gong, Y. et al. Hyperaminoacidemia induces pancreatic α cell proliferation via synergism between the mTORC1 and CaSR-Gq signaling pathways. Nat. Commun. 14, 235 (2023).
Guettier, J.-M. et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc. Natl Acad. Sci. USA 106, 19197–19202 (2009).
Li, J. H. et al. A novel experimental strategy to assess the metabolic effects of selective activation of a Gq-coupled receptor in hepatocytes in vivo. Endocrinology 154, 3539–3551 (2013).
Bone, D. B. J. et al. Skeletal muscle-specific activation of Gq signaling maintains glucose homeostasis. Diabetes 68, 1341–1352 (2019).
Soares, A. G. et al. Stimulation of the epithelial Na(+) channel in renal principal cells by Gs-coupled designer receptors exclusively activated by designer drugs. Front. Physiol. 12, 725782 (2021).
Mironova, E., Suliman, F. & Stockand, J. D. Renal Na(+) excretion consequent to pharmacogenetic activation of G(q)-DREADD in principal cells. Am. J. Physiol. Ren. Physiol. 316, F758–F767 (2019).
Taylor, M. J. et al. Chemogenetic activation of adrenocortical Gq signaling causes hyperaldosteronism and disrupts functional zonation. J. Clin. Invest. 130, 83–93 (2020).
Wan, J. et al. Pancreas-specific CHRM3 activation causes pancreatitis in mice. JCI Insight 6, e132585 (2021).
Makhmutova, M. et al. Pancreatic beta-cells communicate with vagal sensory neurons. Gastroenterology 160, 875–888.e11 (2021).
Rossi, M. et al. Hepatic Gi signaling regulates whole-body glucose homeostasis. J. Clin. Invest. 128, 746–759 (2018).
Kimura, T. et al. Adipocyte G(q) signaling is a regulator of glucose and lipid homeostasis in mice. Nat. Commun. 13, 1652 (2022).
Jain, S. et al. Chronic activation of a designer G(q)-coupled receptor improves beta cell function. J. Clin. Invest. 123, 1750–1762 (2013).
Wood, C. M. et al. Chemogenetics identifies separate area 25 brain circuits involved in anhedonia and anxiety in marmosets. Sci. Transl. Med. 15, eade1779 (2023).
Roseboom, P. H. et al. Evidence in primates supporting the use of chemogenetics for the treatment of human refractory neuropsychiatric disorders. Mol. Ther. 29, 3484–3497 (2021).
Poth, K. M., Texakalidis, P. & Boulis, N. M. Chemogenetics: beyond lesions and electrodes. Neurosurgery 89, nyab147 (2021).
Sternson, S. M. & Bleakman, D. Chemogenetics: drug-controlled gene therapies for neural circuit disorders. Cell Gene Ther. Insights 6, 1079–1094 (2020).
Bowes, J. et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 11, 909–922 (2012).
Conklin, B. R. et al. Engineering GPCR signaling pathways with RASSLs. Nat. Methods 5, 673–678 (2008).
Acknowledgements
Work in the Roth laboratory is supported by grants from the NIH. J.W. was supported by funds from the NIDDK Intramural Research Program (Bethesda, MD, USA). This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIT) (No. 2022R1C1C1008786). T.M. was supported by the Japan Agency for Medical Research and Development (JP18dm0307007).
Author information
Authors and Affiliations
Contributions
Introduction (H.J.K., T.M., J.W. and B.L.R.); Experimentation (H.J.K., T.M., J.W. and B.L.R.); Results (H.J.K., T.M., J.W. and B.L.R.); Applications (H.J.K., T.M., J.W. and B.L.R.); Reproducibility and data deposition (H.J.K., T.M., J.W. and B.L.R.); Limitations and optimizations (H.J.K., T.M., J.W. and B.L.R.); Outlook (H.J.K., T.M., J.W. and B.L.R.); Overview of the Primer (B.L.R.).
Corresponding authors
Ethics declarations
Competing interests
B.L.R. is an SAB member of Onsero, Epiodyne, Escient and Septerna Pharmaceuticals and is listed as an inventor on patents related to the TRUPATH technology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks S. Kiyonaka, J. Neumaier, C. Price and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
DiscoveRX: https://www.discoverx.com/home
Eurofins: https://www.eurofinsdiscovery.com/
Similarity Ensemble Approach: https://sea.bkslab.org
Glossary
- Chemogenetic actuator
-
The ligand activating the designer receptor exclusively activated by designer drug.
- Chemogenetic transducer
-
The receptor construct.
- Codon optimization
-
Experimental approaches designed to improve the codon composition of a recombinant gene without altering the amino acid sequence.
- Kinome
-
A collection of entire kinases.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, H.J., Minamimoto, T., Wess, J. et al. Chemogenetics for cell-type-specific modulation of signalling and neuronal activity. Nat Rev Methods Primers 3, 93 (2023). https://doi.org/10.1038/s43586-023-00276-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00276-1